
United States and the State
of Wisconsin ex rel. Dr. Toby Watson v. Jennifer King-Vassel
Case No. 12-3671, UNited States Court of Appeals for the Seventh Circuit
Case No.
2:11-cv-00236-JPS, United States District Court for the Eastern District of
Wisconsin
See, PsychRights' Medicaid Fraud Initiative Against Psychiatric Drugging of Children & Youth for Background.
On October 23, 2012, the trial court issued an Order dismissing the case on the grounds that the relator (plaintiff on behalf of the government) needed an expert to (a) explain the mysterious black-box like mechanism whereby a prescription written for a Medicaid patient ends up being paid by Medicaid, and (b) what constitutes a medically accepted indication. However, the court ruled in the relator's favor that he qualified for whistleblower status, which is directly the opposite of what happened in the Alaska case, United States ex rel Law Project for Psychiatric Rights v. Matsutani, et al. The dismissal was appealed. James B. (Jim) Gottstein of PsychRights handled the appeal.
On August, 28, 2013, the United States Court of Appeals for the Seventh Circut (7th Circuit) issued its Opinion, reversing the trial court's dismissal. In doing so, the 7th Circuit held (a) off-label prescriptions that do not have "support" in one of three drug references known as "compendia," (not a "medically accepted indication") submitted to Medicaid for payment are (generally) false claims, and (b) doctors knowingly cause the false claims by writing such prescriptions if they know the patient is a Medicaid recipient (unless they come forward with evidence to the contrary). In other words, the 7th Circuit confirmed that doctors writing prescriptions for use in children and youth that are not for a medically accepted are liable under the False Claims Act. This essentially confirms that doctors writing such prescriptions are wittingly or unwittingly participating in the Fraudulent Scheme depicted below at Step 2.
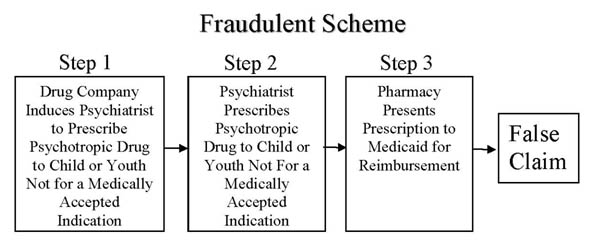
The trial in
ex rel Watson v. King-Vassel was then scheduled to begin
December 9, 2013. However, at the December 3, 2013, Pretrial
Conference, the judge threatened Dr. Watson with a substantial award of
attorneys' fees against him if he went forward and he decided to dismiss the
case. Below is Dr. Watson's Statement regarding his decision to
dismiss the case in the face of this threat.
Court Documents
Appeal
District Court
|
False Claim Medicaid Fraud Lawsuit Settled Yesterday the parties in United States and the State of
Wisconsin ex rel. Dr. Toby Watson v. Jennifer King-Vassel , Case No.
2:11-cv-00236-JPS, U. S. District Court for the Eastern District of
Wisconsin, gave notice to the court that they have settled the qui tam
or "whistleblower" lawsuit against a psychiatrist defendant who wrote
psychiatric prescriptions to a Medicaid child that were not for the
legally defined 'medically accepted indications'. According to the
United States 7th Circuit Court of Appeals which upheld the complaint's
understanding of the federal law, when a prescription is written to a
Medicaid patient and submitted to the state Medicaid program, it is a
claim submitted to the federal government and it is a false claim if
that prescription is not for a use approved by the FDA or supported by
one of the drug references known as compendia that are incorporated into
the Medicaid statute.
Although I hoped to proceed to an actual trial in order to help
children stay off of these harmful drugs, recapture funds for the State
and Federal Medicaid program, and improve the quality of care for the
Medicaid program, due to the judge’s confrontation to my counsel, it
became clear to me there was a serious threat of sanctions and fees
being wrongly put forth in the hopes we would dismiss the lawsuit.
I have therefore decided to dismiss the suit and continue the pursuit
legislatively.
One of the gross concerns was that prior to the settlement, the
Wisconsin Department of Health Services (DHS) issued an affidavit in the
case stating that prescriptions written by the Defendant that were not
for a medically accepted indication and submitted to Medicaid for
payment were not false claims. This is of concern because on
November 5, 2013, nearly the same time, the Wisconsin Attorney General
J.B. Van Hollen's office publically announced on their website that the
State of Wisconsin was going to recapture 7.2 million dollars for the
off-label promotion of one of the drugs actually listed in Watson's qui
tam lawsuit (Risperdal). The Attorney General's office statement
indicated "The manufacturers’ alleged unlawful conduct caused false
and/or fraudulent claims to be submitted to or caused purchases by
government funded health care programs, including the state Medicaid
programs." (italics added).
http://www.doj.state.wi.us/media-center/2013-news-releases/November-5-2013-0
. The way the off-label marketing causes the false claims is by
getting doctors to prescribe drugs for such uses, which then get paid by
Medicaid.
In the Risperdal case, the Federal Department of Justice also
issued a news release on November 4, 2009, stating the total fines and
fees against the makers of Risperdal would be 1.72 billion dollars
because “The conduct at issue in this case jeopardized the health and
safety of patients and damaged the public trust” by marketing their drug
to “elderly nursing home residents, children and individuals with mental
disabilities. The government alleges that J&J and Janssen caused
false claims to be submitted to the federal health care programs by
promoting Risperdal for off-label uses that the federal health care
programs did not cover…” In an earlier case, the Federal Department of Justice issued a news
release on September 2, 2009, indicating Pfizer has agreed to pay 1
billion to resolve allegations under the civil False Claims Act.
This was for partially for the promotion of Geodon, another
anti-psychotic drug, for a non medically accepted indication, whereby
they “caused false claims to be submitted to the government health care
programs for uses that were not medically accepted indications and
therefore not covered by those programs. On December 20th, 2010, the state of Wisconsin’s Attorney General's
office publically stated on its website that it was recovering millions
for the off-label promotion of an epileptic drug, stating it was for
"damages and penalties to compensate Medicaid, a joint funded state and
federal program, and various federal healthcare programs for harm
suffered as a result of its conduct." "The government contends
that Elan...improperly marketed Zonegran for uses not approved by the
FDA, thereby causing the submission of false claims to state Medicaid
programs and to other federally funded health care programs."
This marketing was also for variety of psychiatric conditions.
http://www.doj.state.wi.us/media-center/2010-news-releases/December-20-2010-0
Clearly the Department of Health Services and the Attorney
General’s office of Wisconsin are giving different interpretations of
the federal law that I was able to get very clear interpretation upon by
the 7th District Federal Court. I brought forth this
case for several reasons, foremost being that children should not be
subjected to harmful brain altering drugs that have not been found to
meet the already very relaxed FDA intended use safety standard
and/or the also minimal Medicaid program safety and efficacy standard.
These psychiatric drugs were promoted directly to doctors when the
manufacture knew they were harmful and there could be much less costly
alternatives used. A prescription of Risperdal might cost around
$400, whereas another prescription that could be used in generic form
might cost only $20. We are talking about billions of dollars
spent unnecessarily and illegally and the State of Wisconsin Department
of Health Services and state Medicaid program helped stop this
whistleblower lawsuit. I agree with the Attorney General in that
we need to hold both drug companies and doctors accountable for
their behavior, and certainly hope in the coming weeks I will receive a
return call from the Attorney General, someone who refused to allow me
an appointment and talk to me this past week.
To understand why this is not only a financial problem, according
to a study late in 2009 from Rutgers and Columbia universities, children
covered by Medicaid are given powerful antipsychotic medicines at a rate
four times higher than children whose parents have private insurance.
In 2008, the most recent year for which complete data are available,
Medicaid spent $3.6 billion on antipsychotic medications, up from $1.65
billion in 1999, according to Mathematica Policy Research, a Washington
firm that crunches Medicaid data for HHS. The growth came even as
pharmacy benefits for millions of Medicaid recipients shifted to
Medicare in 2006. We are talking about tens of thousands of children, as
young as 5 months old according to the government report, being given
expensive and dangerous drugs that are being supported to doctors by
misleading and biased drug company researchers. |
3/7/2014
Copyright © 2011-2014 Law Project for Psychiatric Rights. All Rights Reserved