
 |
|
PsychRights' Medicaid Fraud Initiative Against
Psychiatric Drugging of Children & Youth
Model
Medicaid Fraud Complaint
Unsealed Cases
On August 28, 2013, the United States Court of Appeals for the Seventh Circuit issued an Opinion validating the approach set forth here.
The massive psychiatric drugging of America's children, particularly poor, disadvantaged children & youth through Medicaid and in foster care is an unfolding public health catastrophe of massive proportions. This catastrophe is being caused by the fraudulent promotion of these harmful practices by pharmaceutical companies sacrificing children and youth's health, futures and lives on the altar of corporate profits. The Fraudulent Scheme, as it pertains to Medicaid recipients, can be depicted as follows:
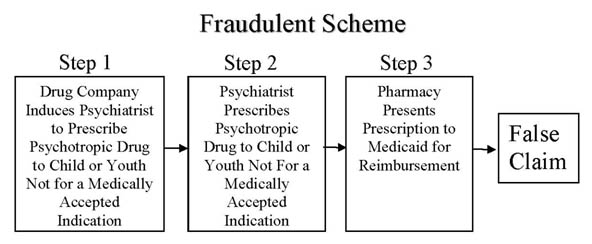
In 2009, Eli Lilly agreed to pay $1.4 Billion in criminal and civil penalties for such off-label promotion of Zyprexa and Pfizer agreed to pay $2.3 Billion for the illegal off-label promotion of Geodon and other drugs. In 2010, Astra-Zeneca agreed to pay $520 million for the illegal off-label promotion of Seroquel for use in children, and Forest Laboratories agreed to pay $309 million for the illegal off-label promotion of the use of Lexapro and Celexa in children. However, despite these large penalties by the drug companies, the practice has not stopped. It is merely a cost of doing business to these pharmaceutical Goliaths and, in fact, caps their liability for these crimes. Most importantly, these settlements have not stopped the practice of child psychiatrists and other prescribers giving these drugs to children and youth and Medicaid continuing to pay for these fraudulent claims.
PsychRights' Medicaid Fraud Initiative Against Psychiatric Drugging of Children & Youth is designed to address this problem by having lawsuits brought against the doctors prescribing these harmful, ineffective drugs, their employers, and the pharmacies filling these prescriptions and submitting them to Medicaid for reimbursement. Once one sues over specific offending prescriptions, all of such prescriptions can be brought in, which means that any psychiatrist on the losing end of such a lawsuit will almost certainly be bankrupted, because each offending prescription carries a penalty of between $5,500 and $11,000. This is why it is expected that once this financial exposure becomes known to the prescribers they will quit the practice. Each prescriber may have a million dollars or few, at most, to lose, but the pharmacies' financial exposure can run into the hundreds of millions of dollars and it is hoped this will attract attorneys to take these cases. Anyone with knowledge of specific offending prescriptions can sue on behalf of the government to recover for such Medicaid Fraud, and receive a percentage of the recovery, if any.
Of course, it can be expected that the defendants will vigorously contest everything, and there are no guarantees of success. However, PsychRights believes what is presented here is accurate. PsychRights has published a YouTube Video and PowerPoint Presentation that goes through the requirements and identifies the major issues.
The Model Qui Tam Complaint PsychRights has put together is set up for former foster youth to sue the doctors who prescribed the drugs to them, their employers, and the pharmacy(ies) submitting the false claims, but it can be easily modified for anyone else to file such a complaint, such as parents, teachers, therapists, etc. PsychRights stands ready to to help people interested in bringing such suits and interested people can e-mail us, or call at (907) 274-7686, or write to 406 G Street, Suite 206, Anchorage, AK 99501.
In 42 USC 1396R-8(k)(3), as relevant here, Congress prohibited reimbursement under Medicaid for any outpatient drugs "used for a medical indication which is not a medically accepted indication." 42 USC 1396R-8(k)(6) then defines "medically accepted indication" as follows:
The term “medically accepted indication” means any use for a covered outpatient drug which is approved under the Federal Food, Drug, and Cosmetic Act [21 U.S.C.A. § 301 et seq.], or the use of which is supported by one or more citations included or approved for inclusion in any of the compendia described in subsection (g)(1)(B)(i) of this section.
42 USC 1396R-8(g)(1)(B)(i), in turn, designates the Compendia as:
(I) American Hospital Formulary Service Drug Information;
(II) United States Pharmacopeia-Drug Information (or its successor publications); and
(III) the DRUGDEX Information SystemAn indication not approved by the FDA is often referred to as "off-label." Congress didn't prohibit reimbursement by Medicaid for all off-label prescriptions, but specifically limited reimbursement for off-label prescriptions to those that have sufficient scientific "support," as documented in one of the Compendia. A couple of illustrations: Geodon is not (yet) approved for any use in children and not supported by any citation in any of the Compendia. Thus, any Geodon prescriptions to children and youth submitted to Medicaid constitute fraud. Similarly, I have seen neuroleptics such as Abilify, Risperdal, Seroquel, & Zyprexa, prescribed for "Oppositional Defiant Disorder," and even for sleep. Such prescriptions are not for "medically accepted indications," and thus automatically constitute Medicaid Fraud.
There are a a lot of technical requirements that must be met, such as the lawsuit must be based on "non-public" information, which in this case is satisfied by having knowledge of offending prescriptions and the cases must initially be filed under seal (in secret).
Under the False Claims Act:
News Releases
News Stories
| February 11, 2010 | Alaska psychiatrists accused of wrongly medicating children: FRAUD CLAIM: Doctors followed drug marketing recklessly, suit says, by Megan Holland, Anchorage Daily News, |
| February 11, 2010 | Lawyer takes on psychiatric industry for over-prescribing foster children, by Rhonda McBride, KTUU |
| March 9, 2010 | FCA Suit Targets Providers, Not Drug Firms, in Novel Off-Label Use Case, Medication Compliance News, March, 2010 |
| June 2, 2010 | Medicating Children: A “Whistleblower’s” Lawsuit Raises a Novel Legal Question, by Robert Whitaker, Mad in America blog, Psychology Today |
| June 7, 2010 | Tracking the American Epidemic of Mental Illness - Part III, by Evelyn Pringle, OpEdNews. |
| June 16, 2010 | Psychotropic Drug Abuse in Foster Care Costs Government Billions, by David Sessions, Politics Daily |
Utah Attorney General's Office Correspondence
- October 22, 2007, letter from Utah Assistant Attorney General David Stallard to the Centers for Medicare & Medicaid Services (Medicaid)
- December 6, 2007, response from Medicaid to Utah Assistant Attorney General David Stallard
- December 17, 2007, follow-up letter from Utah Assistant Attorney General David Stallard to Medicaid
- January 30, 2008, response from Medicaid to Utah Assistant Attorney General David Stallard
Last modified 4/5/2014
Copyright © 2009 -- 2014 Law Project for Psychiatric Rights. All Rights Reserved