|
Nature
Published online: 15
March 2006; | doi:10.1038/440270a
Drug trials: Stacking the deck
Studies of medical literature are confirming what
many suspected — reporters of clinical trials do not always play straight.
Jim Giles talks to those pushing for a fairer deal.
Jim Giles
|


|
|
C.
DARKIN
|
|
They
answer only the questions they want to answer. They ignore evidence that does
not fit with their story. They set up and knock down straw men. Levelled at politicians, such accusations would come as
no surprise. But what if the target were the researchers who test drugs? And
what if the allegations came not from the tabloid press, but from studies
published in prestigious medical journals?
The slurs may
sound over the top, but each is based on hard data. Since 1990, a group of
researchers has met every four years to lay bare the biases that permeate
clinical research. The results make for uncomfortable reading. Although
outright deception is rare, there is now ample evidence to show that our view
of drugs' effectiveness is being subtly distorted. And the motivation, say
the researchers, is financial gain and personal ambition.
"Patients
volunteer for trials, but finances and career motives decide what gets
published," says Peter Gøtzsche, an expert in
clinical trials and director of the Nordic Cochrane Centre in Copenhagen. "This
is ethically indefensible. Change is not easy, but we must get there."
It is a
dramatic conclusion to come from a field of study with no proper name,
staffed by part-time volunteers. Most are journal editors, medical
statisticians or public-health experts, united by fears for the integrity of
clinical trials. For the devotees of 'journalology'
or 'research into research', the literature on clinical trials is their raw
data and patterns of bias are their results.
Some of these
researchers are using their findings to change medical journals and make it
harder for authors to misrepresent results. Others are working on what could
become the biggest reform of clinical-trial reporting for decades: the
creation of a comprehensive international registry of all clinical trials. It
is a powerful idea, which could one day make all trial information public. It
is also an idea that has pitched pharmaceutical companies against advocates
for reform, in a tussle over whether transparency or commercial confidentiality
best serves medical science.
Just say no
One of the
biggest problems with clinical-trial reporting, the suppression of negative
results, shows the importance of such debates. Because clinical researchers
are not obliged to publish their findings, ambiguous or negative results can
languish in filing cabinets, resulting in what Christine Laine,
an editor at the Annals of Internal Medicine in Philadelphia, Pennsylvania,
calls "phantom papers". If that happens, the journal record will
give an over-optimistic impression of the treatments studied, with
consequences for peer reviewers, government regulators and patients.
One alleged
example hit the headlines in 2004. At that time, the antidepressant Paxil (paroxetine), made by
London-based drug giant GlaxoSmithKline, was a popular treatment for
adolescents in the United
States. But doctors have now been warned
off prescribing Paxil to youngsters, after evidence
emerged that it increases the risk of suicidal behaviour.
It was claimed in a court case brought in the United States that
GlaxoSmithKline had suppressed data showing this since 1998. Rick Koenig, a
spokesman for GlaxoSmithKline, says the company thought the charges
unfounded, but agreed to pay $2.5 million to avoid the costs and time of
litigation.
Phantom papers
can be tracked down through trial protocols — the document describing how a
trial will be run and what outcomes will be measured — which have to be
registered with local ethics committees. By matching papers with protocols,
several groups have shown that many trials are completed but not published.
And that, notes Laine, makes it impossible for
journals and health agencies to assess potential drugs. "You never quite
know if other data are out there that would influence your conclusions,"
she says.
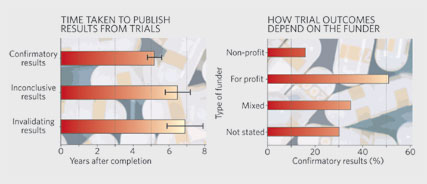
Last year, for
example, a French team showed that only 40% of trials registered with its
country's ethics committees in 1984 had been published by 2002, despite more
than twice as many having been completed1. Crucially, papers with inconclusive
results not only took longer to publish (see graph), they were less likely to
see the light of day at all. Researchers in any field can sit on negative or
inconclusive results. But critics say that clinical researchers carry a
greater ethical burden, as their findings inform decisions about the
licensing of drugs.
Don't
believe the hype
|


|
|
Bitter pill? GlaxoSmithKline denies suppressing data about
antidepressant Paxil's side effects.
J.
RAEDLE/GETTY IMAGES
|
|
Nor
do the problems end when a trial hits an editor's desk. Results from a trial
of the arthritis drug Celebrex (celecoxib)
looked good when they were published in 2000, for example, but less so when
physicians scrutinized the full data set. The original paper, which appeared
in the Journal
of the American Medical Association (JAMA), dismissed fears that Celebrex could cause ulcers. But that was based on data
collected over six months. When other physicians analysed
a full year's worth — which the authors already had at the time of their JAMA submission — they claimed
that Celebrex seemed to cause ulcers just as often
as other treatments2. The original study's authors say that
the later data were too unreliable to be included, but acknowledge that they
could have "avoided confusion" by explaining to editors why they
had omitted them.
This case is
not a one-off. During his PhD at the University of Oxford, UK, epidemiologist
An-Wen Chan looked at the protocols for 122 trials
registered with two Danish ethics committees in 1994–95. More than half of
the outcomes that the protocol said would be measured were missing from the
published paper, he found3. Asked about the missing outcomes, most
authors simply denied the data were ever recorded, despite evidence to the
contrary. And when Chan looked at those missing data, he found that
inconclusive results were significantly more likely to have been left out of
the final publication.
Medical
journals can, however, request such missing data. One idea, adopted by The Lancet three years ago, is to
insist that authors send in a trial protocol when they submit results. That
should help identify whether researchers are reporting all the information
they gathered.
|

|
|
SOURCES:
REF. 1; REF. 4
|
|
But
even with all the data, journal editors face another challenge: hype.
Researchers and sponsors tend to be interested in things that work rather
than those that do not, so authors may subconsciously tweak results and talk
up conclusions. "Researchers are so worried about getting papers
rejected that they put a lot of spin on results to make them seem as exciting
as possible," says Doug Altman, a medical statistician who supervised
Chan at Oxford.
The hype shows
up in a paper's conclusions. In 2003, epidemiologist Bodil
Als-Nielsen and her colleagues at the University of Copenhagen looked at factors that
might influence researchers' conclusions about a drug's efficacy or safety4. Their analysis of 370 trials showed that
the strongest predictor of the authors' conclusions was not the nature of the
data, but the type of sponsor.
|
 Patients volunteer for trials, but
finances and career motives decide what gets published. Patients volunteer for trials, but
finances and career motives decide what gets published. 
|

|
|

|
|

|
Trials
funded by for-profit organizations were significantly more likely to reach a favourable verdict than those sponsored by charities or
governments. Critically, the association was not explained by the papers
having more positive results. In a study under review, Gøtzsche
and his colleagues show that industry-funded meta-analyses — studies that
combine results from several clinical trials of a drug — are similarly prone
to draw positive conclusions that are not supported by the data (see graph).
For many
clinical-trials experts, these funding biases explain all the others. For
each act, be it the suppression of results or the omission of outcomes, there
is a financial motive for the company whose drug is being tested. In many
cases, the company funding the study also employs one or more of the authors.
Given the combination of motive and opportunity, many see drug-company
influence as an inevitably distorting factor.
"When we
see an industry article we get our antennae up," says Steven Goodman, a
medical statistician at Johns
Hopkins University
in Baltimore
and an editor at the Annals of Internal Medicine. "It's not that we
assume the research is done badly. But we have to assume that the company has
done all it can to make its product look as good as possible."
Editorial
control
At JAMA, editors began insisting
last year that all research sponsored by for-profit organizations undergo
independent statistical analysis before acceptance. Cathy DeAngelis,
the journal's editor-in-chief, says JAMA had asked authors to do this for years, but began
requiring it after editors started seeing papers that they thought dishonest.
"People said that for-profit companies would stop sending us
trials," she notes. "Well, guess what? If you look at what we're
publishing you'll see that that's not true."
Still, Goodman
and others caution against blaming everything on industry.
Government-sponsored trials also tend to report positive outcomes5, although the effect is weaker than with
industry studies. And a publication in a big journal can boost authors'
careers as well as company coffers.
|


|
|
JACOB
RIIS
|
|
Others
add that journals must also share the blame. Good peer reviewers and hands-on
editors should, for example, weed out hype. But according to Richard Smith, a
former editor of the BMJ (which was the British Medical Journal) and now head of European
operations for the US
insurer UnitedHealthcare, editors may be biased
towards positive results. In an article published last May, titled
"Medical journals are an extension of the marketing arm of
pharmaceutical companies", he pointed out that reprints of papers
reporting positive results can generate millions of dollars, and that this
might influence editorial decisions6.
There is
certainly evidence that drug companies attempt to use reprint income as a
lever on journals. The Lancet's Richard Horton has said
that authors sometimes contact him to say that sponsors are likely to buy
large numbers of reprints if their study is published. Horton and other
editors at top journals say they rebuff such threats, but some less well
staffed journals lack policies for separating commercial and editorial
decisions, suggesting that reprint income at least has the potential to
distort decisions.
|
 We have to assume that the company
has done all it can to make its product look as good as possible. We have to assume that the company
has done all it can to make its product look as good as possible. 
|

|
|

|
|

|
Merrill
Goozner, who tracks pharmaceutical issues at the
Center for Science in the Public Interest, a lobby group in Washington DC,
agrees. "It's a financial conflict of interest, plain and simple,"
he says.
Full
disclosure
Such
accusations make medical editors angry. They deny that commercial pressures
influence peer review, adding that journals have introduced several measures
that have helped to clean up clinical-trial reporting.
One of the
first initiatives, introduced in 1996 and revised in 2001, is the statement
on Consolidated Standards of Reporting Trials (CONSORT). A set of guidelines
on how to report a clinical trial, the statement is designed to ensure that
authors present results transparently. It seems to be helping. Since top
journals began insisting that authors follow the guidelines, researchers'
descriptions of their methods, for example how they place subjects in
treatment or control groups, have become more accurate7. Such information aids reviewers'
decisions.
|


|
|
S.
GOODMAN
|
|
Journals
have also endorsed trial registries. By registering all trials when they
begin, researchers will find it harder to suppress outcomes, editors believe.
Several registries already exist, including one run by the US
government. The World Health Organization (WHO) is working on an online
portal that would bind these databases into a single source. And in 2004, the
International Committee of Medical Journal Editors announced its members
would not publish the results of trials that had not been placed in a public
registry.
Clinical-trials
experts welcomed the move, but the industry response was patchy. Last June,
the committee was forced to issue another statement after finding that some
sponsors were being deliberately vague and entering terms such as
'investigational drug' in the field for the drug name. A follow-up study
found the quality of information had improved considerably by last October8.
Despite these
successes, advocates of reform say bigger fights lie ahead. The experts
working on the WHO registry want a list of mandatory entries for trial data,
including the primary outcome. For drug companies this is a step too far,
akin to asking an inventor to publish the description of an invention before
it is patented. Instead, the companies propose depositing such information in
a locked database, to be released when the drug obtains marketing approval.
Going public too early, they say, would deter companies from taking risks on
potential treatments and slow down the generation of new drugs.
Yet for
critics such as Smith, even the WHO portal does not go far enough. Along with
other registry advocates, he would like to see all clinical results, not just
protocols and outcomes, published in public databases.
This proposal
would seem to tackle problems with reporting data and bias. But it would not
be simple. Aside from the commercial concerns of the drugs industry, the
creation of a results database could lead to patients pressurizing doctors
for access to experimental medicines. Health insurers and hospitals might
also change the drugs they use after seeing the raw results, rather than
waiting for peer-reviewed papers.
The debate is
in its infancy. Yet clinical-trials experts are more optimistic than they
have been at many points in the past 15 years. Chan, now working for the WHO
registry team in Geneva,
says the first round of consultations with stakeholders should produce a
policy statement in April. He and others add that although industry will
probably continue to resist, the public attention generated by recent
scandals, and the wealth of data available on the problems, mean that time is
ripe for change.
"I'm not
relying on hope," says Gøtzsche. "But the
results of all trials should be made public, not only those that the sponsor
cares to tell the world about. This is incredibly important."
Jim
Giles is a senior reporter for Nature in London.
Article brought
to you by: Nature
|
References
- Decullier, E. , Lhéritier, V. & Chapuis, F. Br. Med. J. 331, 19–22
(2005). | ISI |
- Hrachovec, J.
B. & Mora, M. J. Am. Med. Assoc. 286, 2398
(2001). | Article | ISI | ChemPort |
- Chan, A.-W. , Hróbjartsson,
A. , Haahr, M. T. , Gøtzsche,
P. C. & Altman, D. G. J. Am. Med. Assoc. 291,
2457–2465 (2004). | Article | ISI | ChemPort |
- Als-Nielsen,
B. , Chen, W. , Gluud, C. & Kjaergard, L. L. J. Am. Med. Assoc. 290,
921–928 (2003). | Article | ISI |
- Dickersin, K.
& Min, Y. I. Online J. Curr. Clin. Trials 2, 50 (1993).
- Smith, R. PLoS
Med. 2, e138 (2005). | Article | PubMed |
- Moher, D. ,
Jones, A. & Lepage, L. J. Am. Med.
Assoc. 285, 1992–1995 (2001). | Article | ISI | ChemPort |
- Zarin, D.
A. , Tse, T. & Ide,
N. C. N. Engl. J. Med. 353, 2779–2787
(2005). | Article | PubMed | ISI | ChemPort |
|
|




