Papers
Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up
Rupert McShane, clinical lecturer,a Janet Keene, research assistant,a Kathy Gedling, research assistant,a Christopher Fairburn, professor,b Robin Jacoby, clinical reader,a Tony Hope, reader aa Section of Old Age Psychiatry, University Department of Psychiatry, Warneford Hospital, Oxford OX3 7JX, b University Department of Psychiatry, Warneford Hospital, Oxford, OX3 7JX
Correspondence to: Dr McShane
|
|
Abstract |
|---|
|
|
|---|
Objective: To investigate the contribution of
neuroleptic drugs to cognitive decline in dementia.
Design: Two year prospective, longitudinal study
consisting of interviews every four months, with necropsy follow up.
Setting: Community settings in Oxfordshire.
Subjects: 71 subjects with dementia, initially living
at home with informant.
Main outcome measures: Cognitive function (score
from expanded minimental state examination); behavioural problems (physical
aggression,
hallucinations, persecutory ideas, and disturbance of diurnal rhythm); and postmortem
neuropathological assessment (cortical Lewy body pathology).
Results: The mean (SE) decline in cognitive score
in the 16 patients who took neuroleptics was twice that in the patients
who did not (20.7 (2.9)
v 9.3 (1.3), P=0.002). An increased rate of decline
was also associated with aggression, disturbed diurnal rhythm, and persecutory ideas. However,
only use of neuroleptics and severity of persecutory ideas were independently
associated with
more rapid cognitive decline when all other variables were adjusted for. The start of neuroleptic
treatment coincided with more rapid cognitive decline: median rate of
decline was 5 (interquartile
range 8.5) points per year before treatment and 11 (12) points per year after treatment
(P=0.02). Cortical Lewy body pathology did not account for association
between
neuroleptic use and more rapid decline.
Conclusions: Neuroleptic drugs that are sometimes
used to treat behavioural complications of dementia may worsen already poor
cognitive function.
Randomised controlled trials are needed to confirm a causal relation.
Key messages
|
|
|
Introduction |
|---|
|
|
|---|
Neuroleptic drugs can be of modest benefit in the treatment of behavioural problems in dementia.1 2 No other class of drugs is of superior efficacy1–indeed, only one adequate trial has shown a beneficial effect with a non-neuroleptic drug.3 4 5 The frequency of use of neuroleptics has varied widely,6 7 suggesting that differences in institutional practice contribute substantially to prescribing decisions. In the United States, anxiety about the overuse of neuroleptics in nursing homes has led to federal legislation prohibiting their use for behaviour such as wandering, restlessness, insomnia, or unspecified agitation in the absence of other justifying reasons.8 A recent survey of nursing homes in Glasgow found that 88% of patients taking neuroleptics might be receiving them inappropriately according to the American guidelines.9
The side effects of neuroleptics include the worsening of behavioural disturbance of patients with dementia,10 11 12 falls,13 and fractures.14 The possibility that the cognitive function of patients with dementia may be made worse by neuroleptics has received little attention. Cross sectional surveys have shown that patients with dementia who take neuroleptics have worse cognitive function than those who do not.15 However, this might be because neuroleptics are often prescribed for behavioural problems that are more common in patients with severe dementia. Results from studies comparing cognitive function before and after the use of neuroleptics have been conflicting. Some studies have not shown any effect on global assessments,10 16 17 others have found improvements in memory or orientation,18 19 20 21 22 and some have shown a worsening of cognitive function.11 23
None of these studies examined the long term effects of neuroleptics in dementia or examined the extent to which the effects on cognition were independent of other factors that might affect cognitive function. A more rapid decline in cognitive function has been associated with psychosis,24 25 26 27 hallucinations,28 29 30 31 sleep disturbance, and aggression,31 but no study has adequately controlled for the possibility that this decline may have been due to the neuroleptics used to treat such behaviours.
|
|
Subjects and methods |
|---|
|
|
|---|
Subjects
Through general practitioners and community psychiatric nurses,
we recruited 104
subjects with dementia (Diagnostic and Statistical Manual of Mental
Disorders, third edition, revised) who were living at home with carers who were able
to give a good account of their symptoms. They were assessed every four months. Six
subjects
dropped out after two to five interviews and have been excluded from the analyses. Another 27
patients died within 20 months of entry to the study. The remaining 71 patients were
assessed
on at least six occasions and form the sample for the first multiple regression analysis reported
below. Of these 71 patients, 42 underwent postmortem neuropathological assessment
and were
the sample for the second regression analysis.
Interview
The patients were interviewed every four months and their behaviour
assessed with the
present behavioural examination.32 Physical
aggression,
hallucinations, and persecutory ideas were rated on a seven point frequency scale, relating to the
number of days over the four weeks immediately before assessment on which
the symptom had
occurred. Disturbance of diurnal rhythm that influenced behaviour was rated as present or absent.
Non-cognitive symptoms that were clearly related to temporary physical illness
were rated
as "missing" throughout the study. Cognitive function was assessed with an
expanded version of the minimental state examination.33
Current medication was recorded at each interview. All doses of neuroleptics were converted to a "chlorpromazine equivalent."34 In view of the possible adverse effect of low doses in a subgroup of patients35 we set a low threshold for the definition of substantial neuroleptic use: a mean of 10 mg of chlorpromazine daily over the first 20 months. Our study was conducted before substituted benzamides such as sulpiride were widely used in Britain for treating elderly people. Four drugs accounted for nearly all the neuroleptics prescribed–thioridazine, promazine, haloperidol, and chlorpromazine.
Statistical analysis
The dependent variable in the two multiple regression analyses
was the rate of cognitive
decline over the first six interviews (that is, the coefficient of regression of minimental state
examination score by interview number). In the first analysis, of 71 subjects,
the independent
variables age, sex, duration of dementia, and cognitive score at study entry were entered
simultaneously in a first block. Neuroleptic use and the mean scores over the
six interviews for
hallucinations, persecutory ideas, physical aggression, and disturbance in diurnal rhythm were
then entered stepwise. We repeated the analysis for the group of 42 patients
for whom necropsy
data were available, with the addition to the first block of a dichotomous variable indicating the
presence or absence of cortical Lewy body pathology.
For the purposes of examining the temporal relation between the start of neuroleptic use and the rate of cognitive decline, we performed a third analysis. We identified 20 patients from the total sample of 98 who were not taking neuroleptics at entry to the study but who started them during the study and continued to take them for at least four months and who had not reached the lowest levels of the expanded minimental state examination when they started taking neuroleptics. We compared the decline in their scores over the year before they started taking neuroleptics with the decline in the year after.
We also compared the 20 subjects' decline in the year after starting neuroleptics with that of 20 matched control patients, drawn from the same sample, who never took neuroleptics. We identified each control on the basis that he or she had the same cognitive score (within four points) as that of the matching subject at the interview immediately before starting neuroleptic treatment. In order to reduce the risk of biased selection of those controls who had a larger number of interviews because they survived longer (a factor which was likely to be associated with a slower rate of cognitive decline), the controls were required to have an equivalent cognitive score at the same point after entry to the study as their matching case. We assessed the significance of these comparisons with unpaired tests (Wilcoxon rank sum) because there were some cases who were not followed for the whole two year period–either because they died within a year of starting neuroleptic treatment or because they started treatment less than a year after entering the study.
|
|
Results |
|---|
|
|
|---|
Subjects
Of the 71 patients in our first analysis, 37 were men. The mean
(SE) age at entry to the
was 72.6 (0.7) years. The age of onset of dementia was less than 65 years in 11 patients, and the
mean duration of dementia at study entry was 5.7 (0.5) years. The
mean minimental state
examination score at study entry was 15.5 (0.9), while the mean score for the expanded version
of the examination was 33 (1.6). Of the 42 patients who underwent necropsy,
seven had cortical
Lewy body pathology and 10 took neuroleptics at some point.
Rate of cognitive decline
The decline in the mean (SE) score for the expanded examination
in the patients who took
neuroleptics was twice that in those patients who did not (20.7 (2.9)
v 9.3 (1.3), P=0.002). An increased rate of decline was also
associated with aggression, disturbance of diurnal rhythm, and persecutory ideas (table
1). Furthermore, the patients who were prescribed neuroleptics were
more aggressive and had more severe disturbance of diurnal rhythm (table
2). Table 3 shows the extent
of correlation between the severity of behavioural changes and other factors that might have
predicted a more rapid rate of cognitive decline. There was no correlation
between the rate of
cognitive decline and the dose of neuroleptic (r
=0.19, P=0.4).
|
|
|
None of the variables that were entered at the first step of the multiple regression analysis (age, sex, duration of dementia, and initial cognitive score), either taken together or separately, made a significant contribution to cognitive decline. Similarly, hallucinations, disturbance of diurnal rhythm, and aggression were excluded from the final model. The final model shows that only neuroleptic treatment and the severity of persecutory ideas were associated with increased rate of cognitive decline once the effect of all the other variables was taken into account (table 4). The significance of these variables as independent predictors was unaffected by square root transformation. The amount of the explained variance in cognitive decline that was attributable to the use of neuroleptics was 19.9%.
|
We repeated the analysis for the 42 subjects who underwent necropsy. Cortical Lewy body pathology did not make an independent contribution to more rapid decline. Neuroleptic treatment remained a significant independent predictor of cognitive decline (data not shown).
Rate of decline before and after taking neuroleptics
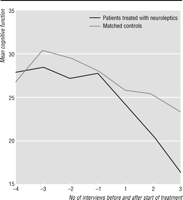
Figure
1 shows the pattern of cognitive decline
in the 20 patients who started taking neuroleptics after study entry and the pattern
of decline in
their 20 matched controls. Among the 20 cases, the median (interquartile range) score for
cognitive function at the interview before neuroleptic treatment was started was
28.5 (18.5). The
median rate of decline in the score over the year before the start of neuroleptic treatment was 5
(8.5) points per year in the 10 cases for whom this information was available.
In the 17 cases who
were followed for at least a year after neuroleptics were started the median rate of decline was
more than twice as fast at 11 (12) points per year. This difference
was significant (W=95,
z=-2.3, P=0.02). The median decline in scores in the matched controls
over the equivalent period was 7.5 (9). This was also significantly lower than the rate of
cognitive decline in those taking neuroleptics (W=377, z=-2.3,
P=0.02).
|
|
|
Discussion |
|---|
|
|
|---|
The main finding in this study was that the use of neuroleptic drugs was associated with an increased rate of cognitive decline in dementia. This association was independent of the degree of dementia and of the behavioural symptoms for which the neuroleptics might have been prescribed. This finding does not, of itself, exclude the possibility that the neuroleptics seemed to cause more rapid decline because patients who were already on a steeper trajectory of cognitive decline were more likely to be prescribed them for reasons other than behavioural problems. However, we also found that the point when patients started treatment with neuroleptics coincided with an increase in their rate of cognitive decline. In addition, we reproduced the findings of others that the rate of cognitive decline is greater in patients who have persecutory ideas.24 26 27 30
Robustness of study
One of the main strengths of our study is that it was longitudinal
in design and that valid,
reliable instruments were used for the collection of behavioural and cognitive data. It was also
possible to compare the rates of decline of patients starting neuroleptic
treatment over a much
longer period than in conventional drug trials. A third advantage was that neuropathological
diagnosis was available in over half of our subjects.
Several methodological weaknesses need to be addressed. First, this study is not a randomised controlled trial of the effect of neuroleptics on cognitive decline. Second, subjects' extrapyramidal function was not assessed. The effect of neuroleptics may have been mediated in some way by impaired extrapyramidal function. Third, our sample may have been less homogeneous than others which were confined to patients with clinical Alzheimer's disease. However, most of our subjects had clinical Alzheimer's disease and most of those with clinical vascular dementia also had Alzheimer's disease at autopsy (data not shown), a finding that concurs with that of Galasko et al.36 Fourth, the number of subjects whose cognitive decline was examined before and after starting neuroleptic treatment was small. This militated against the application of statistical tests to multiple time points. The number of statistical tests applied in this analysis was therefore limited by examining only two time points, a year before and a year after the start of treatment, and conventional levels of significance were applied.
Mechanisms for cognitive decline
Our results do not support the idea that the effect of neuroleptics
on cognitive function
is confined to a subgroup of patients with cortical Lewy body pathology who are particularly
sensitive to the drugs.35 There are several other possible
mechanisms by which neuroleptics might contribute to cognitive decline.
The most parsimonious
explanation is that the anticholinergic effect of the neuroleptics resulted in reduced
attention.12 37 A
second possibility is that both the faster cognitive decline and the persecutory ideas were due to
unidentified episodes of delirium. Changes in cognitive function might also have been
mediated
by changes associated with chronic use of neuroleptics, such as enlargement of the caudate
nucleus,38 39
40 or an increased susceptibility to develop the
neurofibrillary change of Alzheimer's disease41
or impairment of compensatory neurotransmitter responses to neuronal degeneration.42
Our results support the hypothesis that neuroleptics contribute to cognitive decline in people with dementia but do not prove it. Randomised controlled trials are necessary to confirm a causal link. We believe that an appropriate response at present would be to undertake regular review of the need for patients to continue taking neuroleptic drugs, pursuing trials without medication where possible. This study highlights the importance of understanding the neurological basis of behavioural changes in dementia43 so that less toxic drugs can be developed for their treatment.
|
|
Acknowledgements |
|---|
We thank Dr Paul Griffiths for statistical advice and Sandra Cooper for administrative help.
Funding: This work was supported by a Medical Research Council project grant to TH and CF.
Conflict of interest: None.
|
|
References |
|---|
|
|
|---|
- Devanand DP, Sackeim HA, Mayeux R. Psychosis, behavioral disturbance, and the use of neuroleptics in dementia. Compr Psychiatry 1988;29:387-401.
- Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63. [Medline]
- Nyth AL, Gottfries CG, Lyby K, Smedegaard-Andersen L, Gylding-Sabroe J, Kristensen M, et al. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatr Scand 1992;86:138-45. [Medline]
- Schneider LS, Sobin PB. Non-neuroleptic treatment of behavioral symptoms and agitation in Alzheimer's disease and other dementia. Psychopharmacol Bull 1992;28:71-9. [Medline]
- Smith DA, Perry PJ. Non-neuroleptic treatment of disruptive behavior in organic mental syndromes. Ann Pharmacother 1992;26:1400-8.
- Mann AH, Jenkins R, Cross PS, Gurland BJ. A comparison of the prescriptions received by the elderly in long-term care in New York and London. Psychol Med 1984;14:891-7. [Medline]
- Gilleard CJ, Morgan K, Wade BE. Patterns of neuroleptic use among the institutionalised elderly. Acta Psychiatr Scand 1983;68:419-25.
- Elon R, Pawlson LG. The impact of OBRA on medical practice within nursing facilities. J Am Geriatr Soc 1992;40:958-63.
- McGrath AM, Jackson GA. Survey of neuroleptic
prescribing in residents of nursing homes in Glasgow. BMJ 1996;312:611-2.
[Free Full Text] - Gotestam KG, Ljunghall S, Olsson B. A double-blind comparison of the effects of haloperidol and cis(Z)-clopenthixol in senile dementia. Acta Psychiatr Scand Suppl 1981;294:46-53.
- Hamilton LD, Bennett JL. The use of trifluoperazine in geriatric patients with chronic organic brain syndrome. J Am Geriatr Soc 1962;10:140-7.
- Barton R, Hurst L. Unnecessary use of tranquillisers in elderly patients. Br J Psychiatry 1966;112:2036-42.
- Davie JW, Blumenthal MD, Robinson Hawkins S. A model of risk of falling for psychogeriatric patients. Arch Gen Psychiatry 1981;38:463-7.
- Ray WA, Griffin MR, Schaffner W, Baugh DK, Melton LJ. Psychotropic drug use and the risk of hip fracture. N Engl J Med 1987;316:363-9.
- Brown JW, Chobor A, Zinn F. Dementia testing in the elderly. J Nerv Ment Dis 1993;181:695-8.
- Cowley LM, Glen RS. Double-blind study of thioridazine and haloperidol in geriatric patients with a psychosis associated with organic brain syndrome. J Clin Psychiatry 1979;40:411-9.
- Barnes R, Veith R, Okimoto J, Raskind M, Gumbrecht G. Efficacy of antipsychotic medications in behaviorally disturbed dementia patients. Am J Psychiatry 1982;139:1170-4. [Abstract]
- Sugarman AA, Williams H, Alderstein AM. Haloperidol in psychiatric disorders of old age. Am J Psychiatry 1964;120:1190-2.
- Tobin JM, Brousseau ER, Lorenz AA. Clinical evaluation of haloperidol in geriatric patients. Geriatrics 1970;25:119-22.
- Smith GR, Taylor CW, Linkous P. Haloperidol versus
thioridazine for the treatment of psychogeriatric patients: a double-blind clinical trial.
Psychosomatics 1974;15:134-8.
[Abstract/Free Full Text] - Lehmann HE, Ban TA. Comparative pharmacotherapy of the aging psychotic patient. Laval Med 1967;38:588-95X.
- Rosen JH. Double-blind comparison of haloperidol and thioridazine in geriatric outpatients. J Clin Psychiatry 1979;40:17-20.
- Devanand DP, Sackeim HA, Brown RP, Mayeux R. A pilot study of haloperidol treatment of psychosis and behavioral disturbance in Alzheimer's disease. Arch Neurol 1989;46:854-7.
- Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer's disease: prospective analyses from the predictors study. Neurology 1994;44:2300-7. [Abstract]
- Mayeux R, Stern Y, Spanton S. Heterogeneity in dementia of the Alzheimer type. Evidence of subgroups. Neurology 1985;35:453-61. [Abstract]
- Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry 1989;25:39-48.
- Rosen J, Zubenko GS. Emergence of psychosis and depression in the longitudinal evaluation of Alzheimer's disease. Biol Psychiatry 1991;29:224-32. [Medline]
- Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer's disease. II: Disorders of perception. Br J Psychiatry 1990;157:76-81, 92.
- Chui HC, Lyness SA, Sobel E, Schneider LS. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer's disease. Arch Neurol 1994;51:676-81.
- Förstl H, Besthorn C, Geiger-Kabisch C, Sattel H, Scheiter-Gasser U. Psychotic features and the course of Alzheimer's disease: relationship to cognitive, electroencephalographic and computerised tomography findings. Acta Psychiatr Scand 1993;87:395-9.
- Mortimer JA, Ebbitt B, Jun S, Finch M. Predictors of cognitive and functional progression in patients with probable Alzheimer's disease. Neurology 1992;42:1689-96. [Abstract]
- Hope T, Fairburn CG. The present behavioural examination (PBE): the development of an interview to measure current behavioural abnormalities. Psychol Med 1992;22:223-30.
- Wilcock GK, Hope RA, Brooks DN, Lantos PL, Oppenheimer C, Reynolds GP, et al. Recommended minimum data to be collected in research studies on Alzheimer's disease. J Neurol Neurosurg Psychiatry 1989;52:693-700. [Abstract]
- Foster P. Neuroleptic equivalence. Pharm J 1989;243;431-2.
- McKeith I, Fairbairn A, Perry R, Thompson P, Perry E. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992;305:673-8. [Medline]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, et al. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol 1994;51:888-95.
- Drachman DA, Leavitt JB. Human memory and the cholinergic system: a relationship to aging? Arch Neurol 1974;30:113-21.
- Jellinger K. Neuropathologic findings after neuroleptic long-term therapy. In: Roizin L, Shiraki H, Grcevic N, eds. Neurotoxicology. New York: Raven Press, 1977: 25-42.
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994;151:1430-6. [Abstract]
- Figiel GS, Krishnan KR, Doraiswamy PM, Nemeroff CB. Caudate hyperintensities in elderly depressed patients with neuroleptic-induced parkinsonism. J Geriatr Psychiatry Neurol 1991;4:86-9.
- Wisniewski HM, Constantinidis J, Wegiel J, Bobinski M, Tarnawski M. Neurofibrillary pathology in brains of elderly schizophrenics treated with neuroleptics. Alzheimer Dis Assoc Disord 1994;8:211-27.
- Chen CP, Alder JT, Bowen DM, Esiri MM, McDonald B, Hope T, et al. Presynaptic serotonergic markers in community-acquired cases of Alzheimer's disease: correlations with depression and neuroleptic medication. J Neurochem 1996;66:1592-8.
- Esiri MM. The basis for behavioural disturbances in dementia. J Neurol Neurosurg Psychiatry 1996;61:127-30.
Related Articles
- Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial
- Clive
Ballard, Marisa Margallo-Lana, Edmund Juszczak, Simon Douglas, Alan
Swann, Alan Thomas, John O'Brien, Anna Everratt, Stuart Sadler, Clare
Maddison, Lesley Lee, Carol Bannister, Ruth Elvish, and Robin Jacoby
BMJ 2005 330: 874.[Abstract] [Full Text]
- Do neuroleptic drugs hasten cognitive decline in dementia?
- C
Holmes, O Fortenza, J Powell, S Lovestone, Robert Tobiansky, Martin
Blanchard, P W Bentham, S E Goh, E M Gregg, P Madeley, A R A Patel, A P
Taylor, Rupert McShane, Tony Hope, Robin Jacoby, Janet Keene, and
Christopher Fairburn
BMJ 1997 314: 1411.[Extract] [Full Text]
This article has been cited by other articles:
(Search Google Scholar for Other Citing Articles)- Livingston, G, Walker, A E, Katona, C L E, Cooper, C
(2007). Antipsychotics and cognitive decline in Alzheimer's disease: the LASER-Alzheimer's disease longitudinal study.
J. Neurol. Neurosurg. Psychiatry
78: 25-29
[Abstract] [Full text] - Wilkosz, P. A., Miyahara, S., Lopez, O. L., DeKosky, S. T., Sweet, R.
A. (2006). Prediction of psychosis onset in Alzheimer disease: the role
of cognitive impairment, depressive symptoms, and further evidence for
psychosis subtypes.. AJGP
14: 352-360
[Abstract] [Full text] - Aupperle, P.
(2006). Management of aggression, agitation, and psychosis in dementia: Focus on atypical antipsychotics.
AM J ALZHEIMERS DIS OTHER DEMEN
21: 101-108
[Abstract] - Schneider, L. S., Dagerman, K. S., Insel, P. (2005). Risk of Death With
Atypical Antipsychotic Drug Treatment for Dementia: Meta-analysis of
Randomized Placebo-Controlled Trials. JAMA
294: 1934-1943
[Abstract] [Full text] - Mamdani, M., Rapoport, M., Shulman, K. I., Herrmann, N., Rochon, P. A.
(2005). Mental Health-Related Drug Utilization Among Older Adults: Prevalence, Trends, and Costs.
AJGP
13: 892-900
[Abstract] [Full text] - Karim, S., Byrne, E. J.
(2005). Treatment of psychosis in elderly people. Adv. Psychiatr. Treat.
11: 286-296
[Abstract] [Full text] - Ballard, C., Margallo-Lana, M., Juszczak, E., Douglas, S., Swann, A.,
Thomas, A., O'Brien, J., Everratt, A., Sadler, S., Maddison, C., Lee,
L., Bannister, C., Elvish, R., Jacoby, R. (2005). Quetiapine and
rivastigmine and cognitive decline in Alzheimer's disease: randomised
double blind placebo controlled trial. BMJ
330: 874-
[Abstract] [Full text] - Aarsland, D., Andersen, K., Larsen, J. P., Perry, R., Wentzel-Larsen, T., Lolk, A., Kragh-Sorensen, P.
(2004). The Rate of Cognitive Decline in Parkinson Disease. Arch Neurol
61: 1906-1911
[Abstract] [Full text] - Perez-Madrinan, G., Cook, S. E., Saxton, J. A., Miyahara, S., Lopez, O.
L., Kaufer, D. I., Aizenstein, H. J., DeKosky, S. T., Sweet, R. A.
(2004). Alzheimer Disease With Psychosis: Excess Cognitive Impairment
Is Restricted to the Misidentification Subtype. AJGP
12: 449-456
[Abstract] [Full text] - Burns, A., Byrne, J., Ballard, C., Holmes, C.
(2002). Sensory stimulation in dementia. BMJ
325: 1312-1313
[Full text] - Greicius, M D, Geschwind, M D, Miller, B L
(2002). Presenile dementia syndromes: an update on taxonomy and diagnosis.
J. Neurol. Neurosurg. Psychiatry
72: 691-700
[Abstract] [Full text] - Furniss, L.
(2002). Use of medicines in nursing homes for older people. Adv. Psychiatr. Treat.
8: 198-204
[Full text] - Sweet, R. A., Kamboh, M. I., Wisniewski, S. R., Lopez, O. L., Klunk, W. E., Kaufer, D. I., DeKosky, S. T.
(2002). Apolipoprotein E and Alpha-1-Antichymotrypsin Genotypes Do Not Predict Time to Psychosis in Alzheimer's Disease.
J Geriatr Psychiatry Neurol
15: 24-30
[Abstract] - Talerico, K. A., Evans, L. K., Strumpf, N. E.
(2002). Mental Health Correlates of Aggression in Nursing Home Residents With Dementia.
Gerontologist
42: 169-177
[Abstract] [Full text] - Sweet, R. A., Nimgaonkar, V. L., Devlin, B., Lopez, O. L., DeKosky, S. T.
(2002). Increased familial risk of the psychotic phenotype of Alzheimer disease.
Neurology
58: 907-911
[Abstract] [Full text] - Lopez, O L, Becker, J T, Wisniewski, S, Saxton, J, Kaufer, D I, DeKosky, S T
(2002). Cholinesterase inhibitor treatment alters the natural history of Alzheimer's disease.
J. Neurol. Neurosurg. Psychiatry
72: 310-314
[Abstract] [Full text] - Bouman, W. P., Pinner, G.
(2002). Use of atypical antipsychotic drugs in old age psychiatry. Adv. Psychiatr. Treat.
8: 49-58
[Full text] - Ballard, C., O'Brien, J.
(1999). Treating behavioural and psychological signs in Alzheimer's disease.
BMJ
319: 138-139
[Full text] - McKenzie, D. A., Mullooly, J. P., McFarland, B. H., Semradek, J. A., McCamant, L. E.
(1999). Changes in Antipsychotic Drug Use Following Shifts in Policy: A Multilevel Analysis.
Research on Aging
21: 304-337
[Abstract] - Devanand, D.P., Marder, K., Michaels, K. S., Sackeim, H. A., Bell, K.,
Sullivan, M. A., Cooper, T. B., Pelton, G. H., Mayeux, R. (1998). A
Randomized, Placebo-Controlled Dose-Comparison Trial of Haloperidol for
Psychosis and Disruptive Behaviors in Alzheimer's Disease. Am. J. Psychiatry
155: 1512-1520
[Abstract] [Full text] - Callaway, J. T.
(1998). Psychopharmacological Treatment of Dementia. Research on Social Work Practice
8: 452-474
[Abstract] - Holmes, C, Fortenza, O, Powell, J, Lovestone, S, Tobiansky, R.,
Blanchard, M., Bentham, P W, Goh, S E, Gregg, E M, Madeley, P, Patel, A
R A, Taylor, A P, McShane, R., Hope, T., Jacoby, R., Keene, J.,
Fairburn, C. (1997). Do neuroleptic drugs hasten cognitive decline in
dementia?. BMJ
314: 1411-1411
[Full text] - (1997). CAN NEUROLEPTIC DRUGS SPEED COGNITIVE DECLINE IN DEMENTIA?.
JWatch General
1997: 4-4
[Full text]