|
Effectiveness and Cost of Olanzapine and Haloperidol in the
Treatment of Schizophrenia
A
Randomized Controlled Trial
Robert
Rosenheck, MD; Deborah Perlick, PhD; Stephen Bingham, PhD; Wen Liu-Mares,
PhD; Joseph Collins, ScD; Stuart Warren, JD, PharmD; Douglas Leslie, PhD;
Edward Allan, MD; E. Cabrina Campbell, MD; Stanley Caroff, MD; June Corwin,
PhD; Lori Davis, MD; Richard Douyon, MD; Lawrence Dunn, MD; Denise Evans,
MD; Ede Frecska, MD; John Grabowski, MD; David Graeber, MD; Lawrence Herz,
MD; Kong Kwon, MD; William Lawson, MD; Felicitas Mena, MD; Javaid Sheikh,
MD; David Smelson, PhD; Valerie Smith-Gamble, MD; for the Department of
Veterans Affairs Cooperative Study Group on the Cost-Effectiveness of
Olanzapine
JAMA. 2003;290:2693-2702.
ABSTRACT
Context Although olanzapine has been
widely adopted as a treatment of choice for schizophrenia, its
long-term effectiveness and costs have not been evaluated in a
controlled trial in comparison with a standard antipsychotic
drug.
Objective To evaluate the effectiveness
and cost impact of olanzapine compared with haloperidol in the
treatment of schizophrenia.
Design and
Setting
Double-blind, randomized controlled trial with randomization
conducted between June 1998 and June 2000 at 17 US Department of
Veterans Affairs medical centers.
Participants Three hundred nine patients
with a Diagnostic and Statistical Manual of Mental Disorders,
Fourth Edition diagnosis of schizophrenia or schizoaffective
disorder, serious symptoms, and serious dysfunction for the
previous 2 years. Fifty-nine percent fully completed and 36%
partially completed follow-up assessments.
Interventions Patients were randomly
assigned to receive flexibly dosed olanzapine, 5 to 20 mg/d,
with prophylactic benztropine, 1 to 4 mg/d (n = 159); or haloperidol,
5 to 20 mg/d (n = 150), for 12 months.
Main Outcome
Measures
Standardized measures of symptoms, quality of life,
neurocognitive status, and adverse effects of medication.
Veterans Affairs administrative data and interviews concerning
non-VA service use were used to estimate costs from the
perspective of the VA health care system and society as a whole
(ie, consumption of all resources on behalf of these patients).
Results There were no significant
differences between groups in study retention; positive,
negative, or total symptoms of schizophrenia; quality of life;
or extrapyramidal symptoms. Olanzapine was associated with
reduced akathisia in the intention-to-treat analysis (P<.001)
and with lower symptoms of tardive dyskinesia in a secondary
analysis including only observations during blinded treatment
with study drug. Small but significant advantages were also
observed on measures of memory and motor function. Olanzapine
was also associated with more frequent reports of weight gain
and significantly greater VA costs, ranging from $3000 to $9000
annually. Differences in societal costs were somewhat smaller
and were not significant.
Conclusion Olanzapine does not
demonstrate advantages compared with haloperidol (in combination
with prophylactic benztropine) in compliance, symptoms,
extrapyramidal symptoms, or overall quality of life, and its
benefits in reducing akathisia and improving cognition must be
balanced with the problems of weight gain and higher cost.
INTRODUCTION
|

|

|
Schizophrenia is a disabling mental illness that affects more than
2 million persons in the United States1 and was estimated to
consume $16 billion of US health care services in 1990.2
In recent years, a new series of antipsychotic medications has been
released, referred to as "atypical" because they have fewer extrapyramidal
adverse effects than older agents do.3-5
The most widely used of these medications in the treatment of
schizophrenia is olanzapine,6
with $3.7 billion in 2002 worldwide annual sales.7
In a series of randomized trials, olanzapine had fewer extrapyramidal
adverse effects than haloperidol8-10
and, in some studies8,
10-13
but not others,9,
14-15
was associated with greater improvement in symptoms and quality
of life and lower total health care costs.15
However, a recent review of 20 olanzapine trials by the Cochrane
Collaboration5
concluded that "the large proportions of participants
leaving the studies early . . . make it difficult to draw
conclusions on clinical effects. Large long-term randomized trials
. . . are long overdue."
Olanzapine, like
other atypical antipsychotic agents, can cause serious weight
gain16
and may also be associated with hyperglycemia,17
diabetes,18
and hyperlipidemia,19-20
increasing the importance of evaluating its benefits. No
long-term effectiveness study has compared olanzapine or any of
the other atypical antipsychotics except clozapine,21-22
whose use is quite restricted, with a conventional drug.
Although olanzapine is more expensive than conventional agents
(costing >$4000 more annually at wholesale prices6),
if it yields equivalent savings in other health costs, these
expenditures would be justified. To further evaluate the effectiveness
and cost of olanzapine, we conducted a 12-month clinical trial
comparing olanzapine with haloperidol, a widely used
conventional antipsychotic agent. We hypothesized that olanzapine
would outperform haloperidol on 3 primary outcomes, as
demonstrated by fewer symptoms, better quality of life, and lower
costs in patients with schizophrenia.
METHODS
Between June 1998 and June 2000, patients at 17 Department of Veterans
Affairs (VA) medical centers were randomly assigned to
olanzapine or haloperidol. Medication kits were prepared in sets
of 4 (2 olanzapine and 2 haloperidol) and each was labeled with
a random sequence number. Patients were assigned a kit at the
end of a telephone conversation with the coordinating center.
Human rights committees at each participating medical center approved
the protocol and all patients provided written informed consent.
Data from an 18th site were excluded because of problems with a
local institutional review board unrelated to this study.
Entry
Criteria
The study was
initially targeted to patients currently hospitalized for
schizophrenia for less than 365 days, but the criteria were expanded
after 9 months to include patients with schizoaffective disorder
and outpatients with any history of psychiatric hospitalization during
the previous 2 years.
Eligibility criteria
included (1) a Diagnostic and Statistical Manual of Mental
Disorders, Fourth Edition (DSM-IV) diagnosis of
schizophrenia or schizoaffective disorder on the Structured Clinical
Interview for DSM-IV Disorders23;
(2) serious symptoms (ie, score of 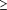 36
on the Brief Psychiatric Rating Scale24);
and (3) serious dysfunction for the previous 2 years with
inability to work or social constriction. Patients were excluded
if they or their clinicians were unable or unwilling to
cooperate; if they had a serious medical illness, unexplained seizures,
or severe medication allergies; or if they had previously participated
in olanzapine research. 36
on the Brief Psychiatric Rating Scale24);
and (3) serious dysfunction for the previous 2 years with
inability to work or social constriction. Patients were excluded
if they or their clinicians were unable or unwilling to
cooperate; if they had a serious medical illness, unexplained seizures,
or severe medication allergies; or if they had previously participated
in olanzapine research.
The medical records
of 4386 patients were reviewed (Figure
1). Only 2141 (49%) were eligible for further assessment;
1530 (35%) either refused participation themselves or their
clinicians refused participation; 7% could not participate for
other reasons; and 309 (7%) provided informed consent and were
randomized.
|
|

|
|
Figure
1.
Enrollment, Allocation, Follow-up, and Analysis
|
|
|
Pharmacotherapy
After completing
baseline assessments, patients were assigned to receive
double-blind treatment with oral olanzapine, 5 to 20 mg/d, or
haloperidol, 5 to 20 mg/d. Dose adjustments were made as
clinically indicated, using 4 fixed dosage levels at 5-mg
intervals. Patients assigned to receive haloperidol also received
prophylactic benztropine mesylate, 1 to 4 mg/d, for extrapyramidal
symptoms (EPS). The olanzapine group received matching placebo
benztropine, and both groups could increase the dose with active
benztropine. The protocol did not allow concomitant use of other
antipsychotic medications, although other psychotropic
medications were permitted.
Psychosocial
Treatment
A predefined program
of psychosocial treatment was offered to both drug treatment
groups through a structured treatment planning process.25
Outcome
Measures
Symptom outcomes were
assessed at baseline, 6 weeks, and 3, 6, 9, and 12 months with
the Positive and Negative Syndrome Scale (PANSS),26
in which high scores reflect worse symptoms and a 20% reduction
represents clinically important improvement (possible range of
scores, 30-210).27
The Heinrichs-Carpenter Quality of Life Scale (QOLS), a
clinician-rated scale, was used to assess social functioning and
severe behavioral deficits, in which higher scores indicate
improvement (possible range, 0-126).28
Secondary outcomes
included adverse effects, assessed with the Barnes scale for
akathisia (ie, restlessness and agitation; possible range, 0-5
[ie, none, questionable, mild, moderate, marked, or severe]),29
the Abnormal Involuntary Movement Scale (AIMS) for tardive
dyskinesia (possible range, 0-42),30
the Simpson-Angus scale for EPS (possible range, 0-4),31
and a checklist of adverse reactions. Further assessment of
clinical status was measured with the Clinical Global Impression
scale32
and quality of life with the Short Form 36-Item Health Survey
(SF-36).33
Neurocognitive status
was assessed at baseline and at 3, 6, and 12 months using the
list learning, recall, recognition, and coding subtests from the
Repeatable Battery for the Assessment of Neuropsychological
Status,34
along with the Grooved Pegboard,35
Wisconsin Card Sorting Test–64 Card Version,36
Trail-Making Test Part B,35
and the Controlled Oral Word Association Test.37
The Wide Range Achievement Test–Revised reading subtest was
used to assess premorbid intellectual functioning.38
Principal components factor analysis with varimax rotation
identified 3 orthogonal factors: motor function, memory, and the
Wisconsin Card Sorting Test. These factors were moderately
intercorrelated (Pearson r range, 0.42-0.58) and together
explained 71% of the variance. They were significantly
correlated with age, sex, education, the Simpson-Angus scale for
EPS, and the Wide Range Achievement Test, which were included as
covariates in analyses of these measures.
Assessment of
Health Care Costs
Health care costs
were calculated by multiplying the number of units of service
for each patient by estimated 1998 unit costs and were estimated
from the perspective of the VA and society as a whole, ie,
consumption of all resources on behalf of these patients.
Societal costs include not only health care costs and criminal
justice costs, for example, but all costs related to these
patients for all payors in society.
Service
Utilization.
Health service data from the VA were derived from national
workload data systems: the patient treatment file (inpatient
care), the extended care file (nursing home and domiciliary care),
and the outpatient care file. The Service Use and Resource Form
recorded patient reports of non-VA medical and mental health inpatient,
residential, and nursing home care and 19 types of medicosurgical
and mental health outpatient care.
VA Unit Costs. Unit costs for VA inpatient
and residential care were estimated on the basis of files
created by the VA's Health Economic Resource Center39-40 using data from the VA's Cost
Distribution Report (CDR). The VA medical and mental health outpatient
unit cost estimates were also derived from the CDR. Group
therapy unit costs were weighted at 20% of the cost of an
individual visit, psychosocial rehabilitation at one third, and
day treatment at half. Costs of intensive case management were
based on cost data from each facility.41
Non–VA Unit Costs. Non-VA costs were derived
from (1) analysis of costs in the 1998 MarketScan data set,42
a compilation of all insurance claims from more than
500 000 private-sector mental health service users; (2) VA
contract payments for private nursing home care available in the
CDR; (3) VA payments for contract residential treatment43;
and (4) published literature presenting unit costs from large
non-VA health care systems.44-45
Medication Costs. The cost of olanzapine was
estimated in a sensitivity analysis using both 1999 discounted
VA pharmacy cost levels of $2.83 per 5 mg6
and wholesale community costs of $4.84 per 5 mg.46
The cost of haloperidol was estimated at $0.02 per 5 mg on the
basis of both VA pharmacy data and community prices.46
Nonstudy medication costs were also estimated using VA and
wholesale prices.
Non–Health Care
Costs.
Non–health care costs were derived from individual interview
data on use of services and from published literature.47-50
These costs included the administrative costs of transfer
payments (eg, disability, welfare),47-48
criminal justice system costs (eg, police contacts, arrests),49-50
and productivity (estimated by employment earnings, included as
a negative cost). For transfer payments, only administrative costs
were included because they alone represent consumed societal resources.47
Statistical
Analyses
The primary analyses
for this study are based on intention-to-treat principles
including all patients as randomized. Power calculations targeted
randomizing 600 patients to yield an 80% chance of detecting a
difference of $8700 in VA inpatient costs. However, only 309
patients were recruited, yielding an 80% chance of detecting a
5-point (6%) difference in symptoms on the PANSS26
or a 5-point (11%) difference in the Heinrichs-Carpenter QOLS.28
Primary clinical
outcomes were analyzed using random-effects repeated-measures
models,51
conducted with PROC MIXED from SAS statistical software, version
8 (SAS Institute Inc, Cary, NC). These models
accommodate correlations among the repeated observations and
therefore allow the inclusion of available data from individuals with
missing observations. Missing data in these models were assumed
to be missing at random. In these models, both group assignment
and time are modeled as class variables, which allows assessment
of both main effects for group assignment (the overall difference
between treatment groups across all time points) and group x time interactions (the difference in slopes).
All models included adjustment for baseline values of the
dependent measures and site effects.
Because cost data
were skewed, both mean and median values of aggregated cost data
are presented, and analyses of statistical significance were
conducted with analysis of covariance of log-transformed measures
and of ranks, controlling for baseline symptoms and service use.
Although 177 patients
(57.3%) discontinued the assigned study medication because of
lack of efficacy, adverse effects, or other reasons (54.1% in
the olanzapine group and 60.7% in the haloperidol group;  21
= 1.37; P = .24), efforts were made to follow up all
patients for a full 12 months and to record nonstudy medications;
26.7% of olanzapine discontinuers and 32.1% of haloperidol
discontinuers were successfully followed up for the entire 12
months ( 21
= 1.37; P = .24), efforts were made to follow up all
patients for a full 12 months and to record nonstudy medications;
26.7% of olanzapine discontinuers and 32.1% of haloperidol
discontinuers were successfully followed up for the entire 12
months ( 21
= 0.35; P = .55). Outcomes were compared first as
randomized (intention-to-treat analysis, for which 63% of all
follow-up data were available) and second after excluding all
data from time points after the first interruption of study drug
use (for which only 49% of all follow-up data were available). An 21
= 0.35; P = .55). Outcomes were compared first as
randomized (intention-to-treat analysis, for which 63% of all
follow-up data were available) and second after excluding all
data from time points after the first interruption of study drug
use (for which only 49% of all follow-up data were available). An
 value
of .05 was used for all statistical tests. value
of .05 was used for all statistical tests.
RESULTS
Sample and Treatment
Patients randomized
to olanzapine (n = 159) and to haloperidol (n = 150) were
significantly different with regard to only 1 measure at
baseline: the PANSS negative subscale (P = .02) (Table
1).
|
|

|
|
Table
1.
Baseline Characteristics of Patients Assigned to Receive Olanzapine
or Haloperidol*
|
|
|
Treatment
During the first 6
weeks of the trial, the mean (SD) dosages were 11.4 (2.2) mg/d
for olanzapine and 11.2 (2.2) mg/d for haloperidol. During the
remainder of the first 6 months, they were 14.7 (3.9) mg/d for
olanzapine and 13.5 (4.4) mg/d for haloperidol and during the
last 6 months were 15.8 (3.9) mg/d for olanzapine and 14.3 (4.6)
mg/d for haloperidol.
Retention
Survival analysis of
participation in the double-blind drug treatment showed no
significant difference between groups (P = .25 by
log-rank test) (Figure
2). There were no significant differences in the proportion
of patients who completed the entire trial while blinded and
receiving study drug (39.3% of patients assigned to haloperidol
vs 45.9% assigned to olanzapine; P = .25) or in the
reasons for discontinuation among those who did not. Patients
assigned to haloperidol were only marginally significantly more
likely to discontinue because of adverse effects (10.0% vs 4%; P
= .08) and there were no significant differences in the
proportion of haloperidol vs olanzapine patients, respectively,
who discontinued because of lack of efficacy or worsening of
symptoms (12.7% vs 17.6%; P = .27); who were lost to
follow-up, missed appointments, or moved (15.3% vs 11.9%; P
= .41); who withdrew consent or were unhappy with blinded treatment
(10.7% vs 8.8%; P = .70); or who discontinued for other
reasons (12.0% vs 11.3%; P = .85). Nor were there significant differences
in the use of concomitant medications at any time, including
conventional antipsychotics (range, 5%-16% for all patients
across time points), nonstudy atypical antipsychotics (5%-17%),
antidepressants (18%-25%), and anticholinergics (6%-11%). On
average, 7.7% of the olanzapine group and 8.6% of the haloperidol group
took open-label anticholinergics.
|
|

|
|
Figure
2.
Retention in Trial
Analyzed by
intention to treat (F = 0.871,204; P = .35).
|
|
|
Outcomes
Fifty-nine percent of
patients fully completed and 36% partially completed follow-up
assessments. Intention-to-treat analysis showed no significant
overall differences during the 12 months of treatment on the
PANSS total symptom score (F1,204 = 0.87; P =
.35) (average difference, -1.1 points; -1.3% favoring olanzapine; Figure
3) or on either the positive (F = 0.221,206; P = .64)
or negative (F1,208 = 1.05; P = .31) subscales.
There were no significant differences at any time point in the
proportion of patients who showed a 20% improvement in PANSS
scores. There was also no significant difference between the
groups on the QOLS (F = 0.141,211; P = .71)
(average difference, 0.1 points; 0.2% favoring olanzapine). Nor
were there any significant differences on specific subscales of
the QOLS that address intrapsychic foundations (F = 0.281,207;
P = .59), interpersonal relationships (F = 0.001,213;
P = .97), or instrumental role functioning (F = 0.01,199;
P = .94); on either the physical (F = 1.941,220; P
= .16) or mental (F = 1.441,216; P = .23) component
scales of a secondary measure of quality of life, the SF-36; or
on a global measure, the Clinical Global Outcomes scale (F =
0.021,196; P = .89). Olanzapine was associated
with significantly lower scores overall on the Barnes scale for
akathisia (F = 14.981,217; P<.001) but not
on the AIMS measure of tardive dyskinesia (F = 1.871,225;
P = .17) or on the Simpson-Angus scale for EPS (F = 0.901,203;
P = .34). Although a smaller proportion of olanzapine patients
had moderate or marked akathisia (5.8% vs 9.6% across all
assessments, with no patient in either group having a severe rating)
(Figure
4), this difference was modest in magnitude.
|
|

|
|
Figure
3.
PANSS Total Scores
PANSS indicates
Positive and Negative Syndrome Subscale. Error bars indicate SDs.
|
|
|
|
|

|
|
Figure
4.
Percentage of Patients With Moderate or Marked Symptoms on Barnes
Akathisia Global Scale
|
|
|
Secondary analysis
excluding observations after the first discontinuation of study
drug also showed no differences on either PANSS symptoms scores
or the QOLS but somewhat more robust overall differences on the
Barnes scale for akathisia (F = 21.01,164; P<.001)
and significant differences on the AIMS (F = 3.951,162; P
= .048).
Because of the
substantial amount of missing data in the later months of the
trial, analysis of variance was used to compare least-square
means at the 6-week and 3-month assessments, controlling for
baseline values. These analyses confirmed the overall analysis, showing
no significant differences on the PANSS (or any of its subscales),
the Simpson-Angus scale for EPS, or the AIMS. The haloperidol
group, however, had significantly higher QOLS scores at 6 weeks
(P = .04) and the olanzapine group had significantly lower
Barnes scale for akathisia scores at both 6 weeks (P = .007)
and 3 months (P<.001).
Intention-to-treat
analysis of neurocognitive test results showed significantly
greater improvement among patients assigned to olanzapine on
tests of motor functioning (F = 6.31,176; P = .02)
and memory (F = 5.21,189; P = .03) but not on the
Wisconsin Card Sorting Test (F = 0.011,186; P
= .93). When observations following interruption of blind study
medication were excluded, these effects were somewhat more
robust for motor functioning (F = 8.31,153; P
= .005) and memory (F = 9.41,163; P = .003), but
the Wisconsin Card Sorting Test remained unimproved (F = 1.091,160;
P = .30). These differences were modest in magnitude, reaching
a maximum of 0.16 SD on motor function and 0.22 SD on memory at
9 months (Figure
5) but were evidently not of sufficient magnitude to improve
overall quality of life, interpersonal relationships, or
instrumental role functioning.
|
|

|
|
Figure
5.
Neurocognitive Assessment
Analyzed by
intention to treat for motor (F = 6.31,176; P =
.02) and memory (F = 5.21,189; P = .03) factors.
Error bars indicate SDs.
|
|
|
Further examination
of adverse events shows that among patients assigned to
olanzapine, there were more frequent reports of weight gain
attributed by the patient as possibly or probably related to
study drug that were marginally significant at 3 months (P
= .07 by Fisher exact test), and significant at 6 months (P
= .002) and 12 months (P = .01) (Table
2). There were fewer reports of restlessness with
olanzapine, reflecting lower levels of akathisia.
|
|

|
|
Table
2.
Adverse Effects Possibly or Probably Attributable to Study Drug*
|
|
|
Service Use
and Cost
There were no
significant differences between treatment groups on any measure
of service use or VA costs, exclusive of medications (Table
3). Total medication costs were 4 to 5 times greater for the
olanzapine group than for the haloperidol group, using VA and wholesale
prices. With the cost of medications included, both total VA
mental health costs and total VA health costs were significantly
greater for patients assigned to olanzapine. The magnitude of
the differences in cost is reduced when medians rather than
means were examined, but nonparametric analysis of ranked cost
data still showed statistically significant differences, with
higher VA costs for olanzapine ranging from $3000 to $9000 across
measures (Table
3).
|
|

|
|
Table
3.
Comparison of 1-Year VA Service Use and Cost Data by
Intention-to-Treat Analysis (n = 309)
|
|
|
Non-VA health costs
and nonhealth costs showed no significant differences, and
differences in societal costs (including both VA and non-VA
costs) were slightly smaller than differences in VA costs and
were not statistically significant. (VA plus non-VA costs were
nonsignificant because while VA costs were significantly
different between groups, non-VA costs were not; when combined,
these costs were less different between groups.) While the costs
of antipsychotic drugs were very different between the groups,
the costs of other psychotropic drugs were the same, which
tended to neutralize the cost difference for antipsychotic agents,
leaving less difference in cost between the 2 groups.
COMMENT
This 12-month double-blind study found no statistically or clinically significant
advantages of olanzapine for schizophrenia on measures of
compliance, symptoms, or overall quality of life, nor did it
find evidence of reduced inpatient use or total cost. Olanzapine treatment
did result in modestly reduced symptoms of akathisia, in less
tardive dyskinesia in one secondary analysis, and in small but
significant improvements in measures of memory and motor
function. Although verbal memory has been reported to be
associated with functional capacity,52
cognitive gains with olanzapine were insufficient to improve
QOLS functioning or employment earnings. Olanzapine was also
associated with more frequent reports of weight gain and with
significantly greater total VA costs, ranging from $3000 to
$9000 per patient annually.
These results are
substantially less favorable for olanzapine than those reported
in previous trials.8-13,15
Perhaps the most unexpected difference was the lack of any
significant advantage for olanzapine on measures of retention,
termination due to adverse effects, or EPS other than akathisia.
These differences are most likely explained by 2 major
differences between this study and others: (1) prophylactic
benztropine was prescribed for the haloperidol group (as
recommended in a recent treatment overview53
and as used in typical clinical practice54)
and (2) outcome data were collected for all patients, even after
interruptions of protocol treatment. Studies more favorable to
olanzapine,8-11,13,
15
in contrast, allowed use of antiparkinsonian agents only after
symptoms arose, increasing the risk of EPS (which is greater for
haloperidol than any other antipsychotic and is especially high
for men55).
Rating biases also may have been introduced in those studies
because without prophylaxis, haloperidol patients can readily be
identified. In addition, since no data were collected after
protocol interruptions due to EPS, there could be no documentation of
eventual recovery from this highly treatable syndrome.
Apparent differences
in symptom and functional outcomes may also reflect these
methodological differences. Clinical descriptions from the
pre-atypical era suggest that even in the absence of frank
pseudoparkinsonian symptoms, patients taking conventional medications
may have akinesia and, as a result, manifest a poor response to
conventional antipsychotics until prescribed anticholinergic agents.56
In the International Collaborative Trial (ICT), one of the
manufacturer's US Food and Drug Administration registration trials
and the basis for most published comparisons of olanzapine and
haloperidol,10-13
66.5% of olanzapine patients but only 46.8% of haloperidol
patients (P<.001) completed 6 weeks of treatment—a
substantial difference that was attributed to lack of efficacy.10
The high failure rate with haloperidol in the ICT, however, may
actually reflect the lack of prophylactic antiparkinsonian
medication. In contrast with the 46.8% retention rate among
haloperidol patients in the ICT, the present study found that
71% of prophylactically treated haloperidol patients were
retained during the first 6 weeks of the trial. Thus, the main
difference between the 2 studies is the far superior performance of
haloperidol in the current trial. Once properly treated for EPS,
haloperidol patients in the ICT would most likely have shown
further clinical improvement, but such improvement was not
documented because data collection was halted. Furthermore, in
the absence of prophylactic treatment, haloperidol patients, like
their raters, could have recognized which treatment they were
receiving, further undoing the double blind.
While the present
study relied on mixed models that used all available data and
associated each observation with the actual time point at which
it was obtained, the ICT relied on a last-observation-carried-forward analysis
in which the last rating during assigned study drug treatment
was used as the single end point, regardless of when it was
obtained. Since patients assigned to olanzapine discontinued later
than haloperidol patients, their last observation was likely to
have been biased by having more time for either improvement or
regression to the mean.
After 6 weeks, the
ICT conducted follow-up assessments only on treatment
responders.10
Reports of reduced long-term health costs13
and improved quality of life11
with olanzapine in the ICT are thus based on seriously biased
last-observation-carried-forward rather than intention-to-treat
analyses and follow-up rates of only 28% over the year for the
olanzapine group and 15% for haloperidol.13
One final difference
is that, unlike the ICT, the current trial did not exclude
patients with current addictive disorders. However, reanalysis
of major outcomes excluding these patients did not reveal any
additional differences in symptoms, adverse effects, or quality
of life.
The major limitations
of this study are the loss of follow-up data, especially in the
later phases of the trial, and the use of concomitant nonstudy
atypical and conventional antipsychotic agents. However, there
were no significant differences between groups in the duration
of adherence to the study protocol, reasons for discontinuing
study drug, or use of any concomitant medications, including
anticholinergic agents. Furthermore, the results based on all
data do not differ from those that exclude data collected after
treatment protocol violations or from analyses limited to the
first 3 months of the trial, when protocol adherence was high.
Also, because the
study sample was overwhelmingly male, all treatment was provided
in VA facilities, and less than 10% of patients considered for
recruitment were enrolled, the generalizability of these
findings to other populations and health care systems is
unknown. The hospitals involved in this trial had somewhat higher
per diem psychiatric inpatient costs than other VA facilities40
but lower per diem costs than non-VA hospitals.44-45
Another possible
limitation is that a strict upper limit of 20 mg/d was placed on
the dosages of both haloperidol and olanzapine. However, the
average dosage of olanzapine used in this study was similar to
the average dosages of 14.1 mg/d nationally in the VA57-58
and to both 12.2 mg/d in a large private sector sample57-58
and dosages reported in the ICT.10
Haloperidol dosages averaged 13.6 mg/d in the current trial
compared with only 11.8 mg/d in the ICT.
Although we did not
meet our power target of 600 patients, we still had 80% power to
detect a 6% difference between groups on the PANSS and an 11%
difference on the QOLS, both notably smaller than generally
accepted difference of 20% needed for clinical significance.
Average differences on both measures were, in fact, less than
2%.
A final limitation is
that this study did not determine whether the benefits of
olanzapine are worth the additional costs and adverse
consequences. It is clear that olanzapine is not a dominant choice
(ie, it does not have both superior outcomes and lower cost).59
Our analyses did not indicate, however, whether the clinically
modest reduction in akathisia and the improvements on
neurocognitive measures are valuable enough to offset the increased
cost of olanzapine and the risk of weight gain and, possibly,
diabetes.18
Although methods have been developed to address this kind of
question,59-61
they are not readily applicable to this study because of the
discrepant positive and negative findings across measures and
because data from a global health utility measure59-60,62
were not collected. However, in view of the very small average
differences between groups in quality of life and the
significantly higher quality-of-life scores in the haloperidol
group at 6 weeks, when adherence to the research protocol was
best, it seems unlikely that olanzapine would have shown
significantly higher scores than haloperidol on such measures.
AUTHOR
INFORMATION
Corresponding Author and Reprints: Robert Rosenheck, MD, Northeast
Program Evaluation Center (182), VA Connecticut Health Care System,
950 Campbell Ave, West Haven, CT 06516 (e-mail: Robert.Rosenheck@yale.edu).
Financial
Disclosures:
Dr Rosenheck has received grant support from Astra-Zeneca and
Bristol-Myers Squibb, both of which manufacture products that
compete with olanzapine in the marketplace, and has received
funds for other research efforts from Lilly. Dr Davis has
received research grants from Lilly. Dr Evans has served as a
consultant to Janssen and Lilly. Dr Herz has served as a
consultant and speaker and has received research grant support
from Lilly.
Author
Contributions:
Dr Rosenheck, as principal investigator, had full access to all
the data in the study and takes responsibility for the integrity
of the data and the accuracy of the data analysis.
Study concept and
design:
Rosenheck, Perlick, Bingham, Collins, Evans.
Acquisition of
data:
Rosenheck, Perlick, Bingham, Warren, Allan, Campbell, Caroff, Corwin, Davis, Douyon, Dunn, Evans,
Frecska, Grabowski, Graeber, Herz, Kwon, Lawson, Mena, Sheikh,
Smelson, Smith-Gamble.
Analysis and
interpretation of data: Rosenheck, Perlick, Bingham, Liu-Mares, Collins,
Leslie.
Drafting of the
manuscript:
Rosenheck, Collins, Kwon, Smelson.
Critical revision
of the manuscript for important intellectual content: Perlick, Bingham, Liu-Mares,
Collins, Warren, Leslie, Allan, Campbell, Caroff, Corwin, Davis, Douyon, Dunn, Evans, Frecska,
Grabowski, Graeber, Herz, Lawson, Mena, Sheikh, Smith-Gamble.
Statistical
expertise:
Rosenheck, Bingham, Liu-Mares, Collins, Leslie.
Obtained funding: Rosenheck, Collins.
Administrative,
technical, or material support: Rosenheck, Perlick, Bingham, Collins, Warren, Corwin, Davis, Dunn, Evans, Grabowski,
Graeber, Lawson, Smith-Gamble.
Study supervision: Rosenheck, Perlick, Collins,
Allan, Davis, Dunn, Grabowski, Kwon, Mena, Sheikh, Smelson.
Members of the
Department of Veterans Affairs Cooperative Study Group on the
Cost-Effectiveness of Olanzapine: Executive Committee: J. Haroldson,
PharmD, BCPS, Albuquerque, NM; J. Buckelew, BS, Albuquerque, NM;
R. Douyon, MD, Miami, Fla; D. Evans, MD, Augusta, Ga; L. Herz,
MD, Bedford, Mass. Data and Safety Monitoring Board: B.
Burns, PhD, Durham, NC; D. Salkever, PhD, Baltimore, Md; C. A.
Tamminga, MD, Baltimore, Md; N. R. Schooler, PhD, Glen Oaks, NY;
R. J. Wyatt, MD, Bethesda, Md; N. M. Laird, PhD, Boston, Mass. Planning
Committee: Dennis Charney, MD, Bethesda, Md. Pharmacy
Activities: Jeffery Haroldson, PharmD, BCPS, Albuquerque, NM;
Jolene Day, Albuquerque, NM; Mike Sather, MS, Albuquerque, NM. Good
Clinical Practices Monitoring Unit: Julia Buckelew, BS,
Albuquerque, NM, Cena Burge, BA, Overland Park, Kan; Pat S.
Manning, RN, MA, Albuquerque, NM; Donna Smith, BS, Montgomery, Ala;
Barbara J. Curtis, Albuquerque, NM. Neuropsychology Training and
Certification Unit: Judith Jaeger, PhD, MPA, Glen Oaks, NY;
Stefanie Berns, PhD, Glen Oaks, NY; Anne Hoff, PhD, Sacramento, Calif.
VA Cooperative Studies Coordinating Center: Anne Horney, BS,
Janie Smith, Cindy Howell. VA Northeast Program Evaluation Center: Michael Sernyak, MD, Jennifer
Cahill, MS, Yuri Agrawal, Joyce Cramer.
Funding/Support: This study was supported by
Lilly, which provided study drug and placebo, and the VA
Cooperative Studies Program.
Role of the Sponsor: Employees of Lilly (Alan
Breier, MD, Robert Obenchain, PhD, and John Kreuger)
participated in the study design and commented on the analyses
and on the manuscript. The analyses and writing of the
manuscript were carried out by the authors independent of the
sponsor.
Author
Affiliations:
Department of Veterans Affairs Medical Center, West Haven, Conn (Drs
Rosenheck, Perlick, Liu-Mares, and Leslie); Department of Psychiatry, Yale
University School of Medicine, New Haven, Conn (Drs Rosenheck, Perlick, Liu-Mares,
and Leslie); Department of Veterans Affairs Cooperative Studies Program
Coordinating Center, Perry Point, Md (Drs Bingham and Collins); Department
of Veterans Affairs Cooperative Studies Program Clinical Research Pharmacy
Coordinating Center (Dr Warren) and Department of Veterans Affairs Medical
Center (Dr Graeber), Albuquerque, NM; Department of Veterans Affairs
Medical Center, Montrose, NY (Dr Allan); Department of Veterans Affairs
Medical Center, Philadelphia, Pa (Drs Campbell and Caroff); Department of
Veterans Affairs Medical Center, New York, NY (Dr Corwin); Department of
Veterans Affairs Medical Center, Tuscaloosa, Ala (Dr Davis); Department of
Veterans Affairs Medical Center, Miami, Fla (Dr Douyon); Department of
Veterans Affairs Medical Center, Durham, NC (Dr Dunn); Department of
Veterans Affairs Medical Center, Augusta, Ga (Dr Evans); Department of
Veterans Affairs Medical Center, Bay Pines, Fla (Dr Frecska); Department of
Veterans Affairs Medical Center, Detroit, Mich (Dr Grabowski); Department
of Veterans Affairs Medical Center, Bedford, Mass (Dr Herz); Department of
Veterans Affairs Medical Center, Brecksville, Ohio (Dr Kwon); Howard
University Medical School, Washington, DC (Dr Lawson); Department of
Veterans Affairs Medical Center, Tuskegee, Ala (Dr Mena); Department of
Veterans Affairs Medical Center, Palo Alto, Calif (Dr Sheikh); Department
of Veterans Affairs Medical Center, Lyons, NJ (Dr Smelson); and Department
of Veterans Affairs Medical Center, Indianapolis, Ind (Dr Smith-Gamble).
REFERENCES
1.
Bromet EJ, Dew MA, Eaton W. Epidemiology of psychosis with special
reference to schizophrenia. In: Tsuang MT, Tohen M, Zahner GE, eds. Textbook
in Psychiatric Epidemiology. New York, NY: John Wiley & Sons; 1996.
2.
Rice DP, Miller LS. The economic burden of schizophrenia: conceptual and
methodological issues, and cost estimates. In: Moscarelli M, Rupp A,
Sartorius N, eds. Handbook of Mental Health Economics and Health Policy,
Volume 1: Schizophrenia. New York, NY: John Wiley & Sons; 1996.
3.
Kerwin RW. The new atypical antipsychotics: a lack of extrapyramidal
side-effects and new routes in schizophrenia research. Br J Psychiatry.
1994;164:141-148. ISI | MEDLINE
4.
Geddes J, Freemantle N, Harrison P, Bebbington P, for the National
Schizophrenia Guideline Development Group. Atypical antipsychotics in the
treatment of schizophrenia: a systematic overview and meta regression
analysis. BMJ. 2000;321:1371-1376. ABSTRACT/FULL TEXT
5.
Duggan L, Fenton M, Dardennes RM, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane
Database Syst Rev. 2003;1:CD001359. MEDLINE
6.
Rosenheck RA, Leslie DL, Sernyak ME. From clinical trials to real-world
practice: use of atypical antipsychotic medication nationally in the
Department of Veterans Affairs. Med Care. 2001;39:302-308. CrossRef | ISI | MEDLINE
7.
Jewell M. Zyprexa takes over Prozac's reign at Eli Lilly. Available at: http://www.courier-journal.com/business/news2003/04/22/biz-3-lil22-6905.html.
Accessed April 25, 2003.
8.
Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S.
Olanzapine versus placebo and haloperidol: acute phase results of the North
American double-blind olanzapine trial. Neuropsychopharmacology.
1996;14:111-123. CrossRef | ISI | MEDLINE
9.
Beasley CM Jr, Hamilton SH, Crawford AM, et al. Olanzapine versus
haloperidol: acute phase results of the international double-blind olanzapine
trial. Eur Neuropsychopharmacol. 1997;7:125-137. CrossRef | ISI | MEDLINE
10.
Tollefson GD, Beasley CM Jr, Tran PV, et al. Olanzapine versus haloperidol
in the treatment of schizophrenia and schizoaffective and schizophreniform
disorders: results of an international collaborative trial. Am J
Psychiatry. 1997;154:457-465. ABSTRACT
11.
Revicki LA, Genduso LA, Hamilton SH, Beasley CM Jr. Olanzapine versus
haloperidol in the treatment of schizophrenia, schizoaffective and
schizophreniform disorders: quality of life outcomes of a randomized
clinical trial. Qual Life Res. 1999;8:417-426. CrossRef | ISI | MEDLINE
12.
Hamilton SH, Edgell ET, Revicki LA, Breier A. Functional outcomes in
schizophrenia: a comparison of olanzapine and haloperidol in a European
sample. Int Clin Psychopharmacol. 2000;15:245-255. ISI | MEDLINE
13.
Hamilton SH, Revicki DA, Edgell ET, Genduso LA, Tollefson G. Clinical and
economic outcomes of olanzapine compared with haloperidol for schizophrenia—results
from a randomised clinical trial. Pharmacoeconomics.
1999;15:469-480. ISI | MEDLINE
14.
Conley RR, Tamminga CA, Bartko JJ, et al. Olanzapine
compared with chlorpromazine in treatment-resistant schizophrenia. Am J
Psychiatry. 1998;155:914-920. ABSTRACT/FULL TEXT
15.
Hamilton SH, Revicki DA, Genduso LA, Beasley CM Jr. Olanzapine versus
placebo and haloperidol: quality of life and efficacy results of the North
American double-blind trial. Neuropsychopharmacology. 1998;18:41-49. ISI | MEDLINE
16.
Casey DE, Zorn SH. The pharmacology of weight gain with antipsychotics. J
Clin Psychiatry. 2001;62(suppl 7):4-10. MEDLINE
17.
Lindenmayer JP, Nathan AM, Smith RC. Hyperglycemia associated with the use
of atypical antipsychotics. J Clin Psychiatry. 2001;62(suppl 23):30-38.
MEDLINE
18.
Sernyak MJ, Leslie D, Alarcon R, Losonczy M, Rosenheck RA. Association of
diabetes mellitus with use of atypical neuroleptics in the treatment of
schizophrenia. Am J Psychiatry. 2002;159:561-566. ABSTRACT/FULL TEXT
19.
Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin,
and blood lipids in olanzapine-treated patients with schizophrenia or
related psychoses. J Clin Psychiatry. 2000;61:742-749. ISI | MEDLINE
20.
Meyer J. Novel antipsychotics and severe hyperlipidemia. J Clin
Psychopharmacol. 2001;21:369-374. CrossRef | ISI | MEDLINE
21.
Rosenheck RA, Cramer J, Xu W, et al, for the Department of Veterans Affairs
Cooperative Study Group on Clozapine in Refractory Schizophrenia. A
comparison of clozapine and haloperidol in the treatment of hospitalized
patients with refractory schizophrenia. N Engl J Med.
1997;337:809-815. ABSTRACT/FULL TEXT
22.
Essock SM, Frisman LK, Covell NH, Hargreaves W.
Cost-effectiveness of clozapine compared with conventional antipsychotic
medications for patients in state hospitals. Arch Gen Psychiatry.
2000;57:987-994. ABSTRACT/FULL TEXT
23.
First MB, Spitzer RL, Gibbon MB, Williams JB. Structured
Clinical Interview for Axes I and II DSM-IV Disorders—Patient Edition
(SCID-I/P). New York, NY: Biometrics Research
Institute, New York State Psychiatric Institute; 1996.
24.
Overall JE, Gorham DR. The Brief Psychiatric Rating
Scale. Psychol Rep. 1962;10:799-812. ISI
25.
Rosenheck R, Tekall J, Peters J, et al. Does participation in psychosocial
treatment augment the benefit of clozapine? Arch Gen Psychiatry.
1998;55:618-625. ABSTRACT/FULL TEXT
26.
Kay SR, Fiszbein A, Opler LA. The Positive and Negative
Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull.
1987;13:261-276. ISI | MEDLINE
27.
Cramer JA, Rosenheck R, Xu W, Henderson W, Thomas J, Charney D. Detecting
improvement in quality of life and symptomatology in schizophrenia. Schizophr
Bull. 2001;27:227-235. ISI | MEDLINE
28.
Heinrichs DW, Hanlon ET, Carpenter WT. The Quality of Life Scale: an
instrument for rating the schizophrenic deficit syndrome. Schizophr
Bull. 1984;10:388-398. ISI | MEDLINE
29.
Barnes TR. A rating scale for drug induced akathisia. Br J Psychiatry.
1989;154:672-676. ABSTRACT
30.
Guy W. Abnormal involuntary movements. In: Guy W, ed. ECDEU Assessment
Manual for Psychopharamcology. Rockville, Md: National Institute of Mental
Health; 1976.
31.
Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta
Psychiatr Scand Suppl. 1970;212:11-19. MEDLINE
32.
Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, Md: US Dept of Health,
Education, and Welfare; 1976:217-222. Publication ADM 76-338.
33.
Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and
Interpretation Guide. Boston, Mass: Health Institute, New England Medical Center; 1993.
34.
Randolph C. Repeatable Battery for the Assessment of
Neuropsychological Status. Odessa, Fla: Psychological Assessment
Resources Inc; 1997.
35.
Reitan RM. Manual for the
Administration of Neuropsychological Test Batteries for Adults and Children.
Tucson, Ariz: Neuropsychology Press; 1979.
36.
Heaton RK. A Manual for the Wisconsin Card Sorting Test. Odessa, Fla: Psychological Assessment
Resources Inc; 1981.
37.
Benton AL, Hamsher K. Multilingual
Aphasia Examination. Iowa City: Department of Neurology,
University of Iowa Hospitals; 1978.
38.
Jastak S, Wilkinson GS. Wide Range Achievement Test—Revised:
Administration Manual. Wilmington, Del: Jastak Assoc; 1984.
39.
Barnett PG. Review of methods to determine VA health care costs. Med
Care. 1999;37(suppl VA):AS9-AS17. ISI | MEDLINE
40.
Wagner TH, Chen S, Yu W, Branett PG. HERCs Inpatient Average Cost Data
Sets for VA Care: Fiscal Years 1998 and 1999. Palo Alto, Calif: Health Economics Resource Center; 2001.
41.
Neale M, Rosenheck R, Baldino R, Cavallaro L. Intensive Psychiatric
Community Care (IPCC) in the Department of Veterans Affairs: Second
National Performance Monitoring Report. West Haven, Conn: Northeast Program Evaluation Center; 1999.
42.
Leslie D, Rosenheck RA. Shifting to outpatient care? mental health
utilization and costs in a privately insured population. Am J
Psychiatry. 1999;156:1250-1257. ABSTRACT/FULL TEXT
43.
Kasprow WJ, Rosenheck RA, Chapdelaine J, Carter R. Health Care for
Homeless Veterans Programs: Twelfth Progress Report. West Haven, Conn: Northeast Program Evaluation Center; 1999.
44.
Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary
medical care with addiction treatment: a randomized controlled trial. JAMA.
2001;286:1715-1723. ABSTRACT/FULL TEXT
45.
Dewan M. Are psychiatrists cost-effective? an analysis of integrated versus
split treatment. Am J Psychiatry. 1999;156:324-326. ABSTRACT/FULL TEXT
46.
Drug Topics Red Book. Montvale, NJ: Medical Economics Co; 1999.
47.
Frisman LK, Rosenheck RA. How transfer payments are treated in
cost-effectiveness and cost-benefit analysis. Adm Policy Ment Health.
1996;23:533-546. ISI
48.
Schobel BD. Administrative expenses under OASDI. Soc Secur Bull.
1981;44:21-28. ISI | MEDLINE
49.
US Department of Justice. Office
of Justice Programs, Bureau of Justice Statistics. Justice Expenditures
and Employment in the United States; 1988. Washington, DC: US Government Printing
Office; 1991.
50.
US Department of Justice.
Maguire K, Flanagan T, eds. Sourcebook of Criminal Justice
Statistics—1990 (NCJ-130580). Washington, DC: US Government Printing
Office; 1991.
51.
Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical
issues in analysis of longitudinal psychiatric data. Arch Gen
Psychiatry. 1993;50:739-750. ABSTRACT
52.
Green MF. What are the functional consequences of neurocognitive deficits
in schizophrenia? Am J Psychiatry. 1996;153:321-330. ABSTRACT
53.
Kane JM. Schizophrenia. N Engl J Med. 1996;334:34-41. FULL TEXT
54.
Buchanan RW, Kreyenbuhl J, Zito JM, Lehman A. Relationship of the use of
adjunctive pharmacological agents to symptoms and level of function in
schizophrenia. Am J Psychiatry. 2002;159:1035-1043. ABSTRACT/FULL TEXT
55.
Keepers GA, Clappison VJ, Casey DE. Initial anticholinergic prophylaxis for
neuroleptic-induced extrapyramidal syndromes. Arch Gen Psychiatry.
1983;40:1113-1117. ABSTRACT
56.
Klein DF, Davis JM. Diagnosis and
Treatment of Psychiatric Disorders. Baltimore, Md: Williams & Wilkins;
1969:165.
57.
Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults:
a review of current evidence. Harv Rev Psychiatry. 2002;10:280-291. CrossRef | ISI | MEDLINE
58.
Rosenheck RA, Doyle J, Leslie D, Fontana A. Changing environments and
alternative perspectives in evaluating the cost-effectiveness of new
antipsychotic drugs. Schizophr Bull. 2003;29:81-93. ISI | MEDLINE
59.
Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost Effectiveness in
Health and Medicine. New York, NY: Oxford University Press; 1996.
60.
Rosenheck R, Cramer J, Xu W, et al. Multiple outcome assessment in a study
of the cost-effectiveness of clozapine in the treatment of refractory
schizophrenia. Health Serv Res. 1998;33:1237-1261. ISI | MEDLINE
61.
Rosenheck RA, Kasprow W, Frismn LK, Liu-Mares W. Cost-effectiveness of
supported housing for homeless persons with mental illness. Arch Gen
Psychiatry. 2003;60:940-951. ABSTRACT/FULL TEXT
62.
Pyne JM, Sullivan G, Kaplan R, Williams DK. Comparing the sensitivity of
generic effectiveness measures with symptom improvement in persons with
schizophrenia. Med Care. 2003;41:208-217. CrossRef | ISI | MEDLINE
RELATED
ARTICLES IN JAMA
This Week in JAMA
JAMA. 2003;290:2635.
FULL TEXT
|