 A commonly-prescribed antipsychotic drug shrinks the brain within hours of administration.Jim Wehtje / Photodisc / Getty Images
A commonly-prescribed antipsychotic drug shrinks the brain within hours of administration.Jim Wehtje / Photodisc / Getty ImagesA leading antipsychotic drug temporarily reduces the size of a brain region that controls movement and coordination, causing distressing side effects such as shaking, drooling and restless leg syndrome.
Just two hours after injection with haloperidol, an antipsychotic commonly prescribed to treat schizophrenia, healthy volunteers experienced impaired motor abilities that coincided with diminished grey-matter volume in the striatum1 — a brain region that mediates movement.
"We've seen changes in the brain before, but to see significant remodelling of the striatum within a couple of hours is staggering," says Clare Parish at the Howard Florey Institute for brain research in Melbourne, Australia, who was not involved in the study. "Our viewpoint was that only chemical changes would happen in such a short time."
In functional magnetic resonance imaging (fMRI) scans the authors observed the participants' striatal volume diminishing and changes to the structure of the motor circuitry in their brains. Further, their reaction times slowed in a computer test taken after the treatment, indicating the onset of lapses in motor control that affect many patients on antipsychotics.
Dopamine downsizing
Haloperidol has a number of side effects, although many of these are minor and recede within weeks of starting treatment. With few better alternatives, psychiatrists have prescribed the drug for more than 40 years to treat people suffering from hallucinations, delirium, delusions and hyperactivity.
Like most antipsychotics, haloperidol blocks the D2 receptor, which is sensitive to dopamine. The drug stifles the elevated dopamine activity that is thought to underlie psychosis. D2 receptors are abundant in the striatum, where their activity regulates gene expression. But, until now, no one knew that blocking the receptors would rapidly alter the brain's physical structure.
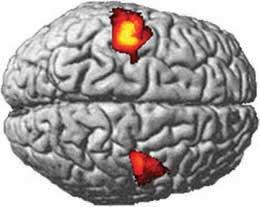 Haloperidol shrank volunteers' striatums in two hours, but they bounced back within a day.Tost, H et al.
Haloperidol shrank volunteers' striatums in two hours, but they bounced back within a day.Tost, H et al."This is the fastest change in brain volume ever seen," says Andreas Meyer-Lindenberg, professor of psychiatry and psychotherapy at the University of Heidelberg in Mannheim, Germany, and a lead author on the report in Nature Neuroscience1. "Studies have found that the volume of brain regions changes over a number of days, but this is in one to two hours, and in half that time it bounces back."
Within a day, volunteers' brains returned to almost their original size as the effects of the single haloperidol dose subsided. Meyer-Lindenberg says this result should alleviate fears that the drug destroys brain cells. "We know it's not killing neurons because the brain rebounds," he says.
Instead, the team suggests that haloperidol downsizes synapses, the junctions through which neurons communicate. Meyer-Lindenberg speculates that the change is mediated by the protein BDNF, which is involved in synapse growth and diminishes in response to antipsychotic treatments in mice.
The bigger picture
"I think this study will cause worry to some," says Shitij Kapur, dean of the Institute of Psychiatry at King's College London. To counteract those fears, he notes that the brain changes caused by the drug seem to be reversible and that the dose used in the study was a little higher than that usually given to patients who had not taken the drug before.
The findings may also hint at why people with bipolar disorder have reduced grey matter in parts of their brains after manic mood swings2. Andrew McIntosh at the University of Edinburgh, UK, says that the connection between the brain-shrinking effects of an antipsychotic reported in this study and the grey-matter reduction he and others have observed in schizophrenic and bipolar patients is "a bit uncertain but this paper definitely makes this worthy of further investigation".
Furthermore, D2 receptors in parts of the striatum have been associated with addiction. This has led Parish to ponder on whether structural changes underlie reward-seeking behaviours. "You wonder what sort of acute changes happen through those receptors in the addicted brain because you hear of cases where addiction happens after just one exposure," she says.
-
References
- Tost, H. et al. Nature Neurosci. doi:doi:10.1038/nn.2572 (2010).
- Moorhead, T. et al. Biol. Psychiatry 62, 894-900 (2007). | Article | PubMed | OpenURL
