Federal health officials have launched a probe into the use of antipsychotic drugs on children in the Medicaid system, amid concern that the medications are being prescribed too often to treat behavioral problems in the very young.
The inspector general's office at Department of Health and Human Services says it recently began a review of antipsychotic-drug use by Medicaid recipients age 17 and under. And various agencies within HHS are requiring officials in all 50 states to tighten oversight of prescriptions for such drugs to Medicaid-eligible young people.
The effort applies to a newer class of antipsychotic drugs known as "atypicals," which include Abilify, the nation's No. 1 prescription drug by sales. The drugs were originally developed to treat psychoses such as schizophrenia, but some now have Food and Drug Administration approval for treatment of children with conditions such as bipolar disorder and irritability associated with autism.
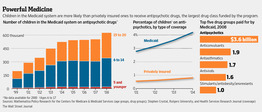
In 2008, the most recent year for which complete data are available, Medicaid, the government health program for the poor, spent $3.6 billion on antipsychotic medications, up from $1.65 billion in 1999, according to Mathematica Policy Research, a Washington firm that crunches Medicaid data for HHS. The growth came even as pharmacy benefits for millions of Medicaid recipients shifted to Medicare in 2006.
Medicaid spends more on antipsychotics than on any other class of drugs. Abilify, made by Otsuka Pharmaceutical Co., appears on lists of the top 10 drugs paid for by Medicaid in various states.
Mark Duggan, a professor and health-policy expert at the University of Pennsylvania's Wharton School, says his analysis of 2010 data on five leading antipsychotics suggests that more than 70% of the cost of these drugs was paid for by Medicaid and other government programs.
The number of people under age 20 receiving Medicaid-funded prescriptions for antipsychotic drugs tripled between 1999 and 2008, according to an analysis by Mathematica.
Dr. Stephen Cha, a chief medical officer at the Centers for Medicare & Medicaid Services, the HHS agency that foots some of the bill for drugs prescribed to Medicaid recipients, says the government wants to reduce what he termed "the unnecessarily high utilization of antipsychotics." He urges doctors to consider other approaches, including therapy to help children and families cope with psychological trauma that could be at the root of behavior issues.
The drugs in question—in addition to Abilify, the brand names include Risperdal, Seroquel and Zyprexa—were developed to replace medications dating to the 1950s such as Haldol and Thorazine, which produced severe side effects such as uncontrollable muscle twitching. The atypicals, introduced in the 1990s and early 2000s, were hailed as safer and more tolerable, and sales grew rapidly.
The FDA's approval of some of the new drugs to treat certain pediatric conditions, coupled with concern about possible side effects on young people and growing off-label use by doctors to treat various forms of violent or aggressive behavior, has sparked debate about whether they are being dispensed too freely to troubled children.
Spokespeople for the makers of Seroquel, AstraZeneca AZN +0.49% PLC, and of Abilify said those drugs should be used for FDA approved indications. Janssen Pharmaceuticals Inc., a unit of Johnson & Johnson JNJ -0.39% that makes Risperdal, noted that the drug had been approved for a number of pediatric uses. Eli Lilly & Co., LLY -0.24% maker of Zyprexa, says the drug's label guides doctors to weigh the risks and consider therapy as part of the treatment. None commented on the government efforts to reduce antipsychotic use by children in the Medicaid system.
Dr. Fernando Siles, a pediatric psychiatrist in the Dallas area who treats many poor foster children, says he sometimes prescribes such medications to treat serious behavior problems. "A child that continues to be aggressive will be kicked out from his foster home," he says. "The antipsychotic is to stabilize the behavior of the child, to keep him from being moved and moved again."
Some doctors say there is too much emphasis on medicating children instead of working with them and their caregivers to understand what is triggering their behavior. Dr. Glenn Saxe, chairman of child and adolescent psychiatry at NYU-Langone Medical Center and a proponent of trauma-focused therapy, says psychiatry has missed "big opportunities to help children. This problem has led to kids being medicated more and more."
Dr. Siles agrees that lots of children could be helped by trauma-centered therapy, "but there is no budget for it."
Children on Medicaid are prescribed antipsychotics at four times the rate of privately insured children, according to a study by Stephen Crystal, a professor of health policy at Rutgers University, that looked at data from 2004 on 6- to 17-year-old children in seven states.
The probe by the inspector general, Daniel Levinson, has been under way for several months and focuses on the five largest Medicaid states: California, Florida, Illinois, New York and Texas. It covers a six-month period from January to June 2011, when 84,654 children age 17 and under in those states received prescriptions for antipsychotics paid for by Medicaid. Pediatric psychiatrists will examine about 700 cases, say people familiar with the effort.
"Through medical-record reviews, we will determine whether these prescriptions were medically indicated, and whether taxpayers were being billed for inappropriate, poor-quality care," says Mr. Levinson.
Government Medicaid data indicate that some of the prescriptions are being written for very young children. An analysis by Mathematica found that in 2008, 19,045 children age 5 and under were prescribed antipsychotics through Medicaid, 3% of recipients under 20, up from 7,759 in 1999, according to James Verdier, a senior fellow at the organization.
Data from the inspector general's five-state probe indicate that 482 children 3 and under were prescribed antipsychotics during the period in question, including 107 children 2 and under. Six were under a year old, including one listed as a month old. The records don't indicate the diagnoses involved.
All five states said they have guidelines to prevent the improper use of the drugs on children in Medicaid.
In New York, a spokesman for the state health department said some children between ages 1 and 2 received antipsychotics for conditions such as autistic disorder and attention deficit disorder with hyperactivity.
Texas said about five children under the age of 1 had been prescribed antipsychotics during the time period of the probe, including two who were five months old.
"No child is getting these drugs unless they're under a doctor's care, and the doctor has to be able to defend the use of the drug," said a spokeswoman for the Texas Health and Human Services Commission. "For infants, the drugs aren't being used for behavior. The infants most often have seizures or complex health issues like heart and respiratory problems, and these drugs can be prescribed to help with discomfort."
Of particular concern is use of the drugs on foster children in the Medicaid system. One study, based on 2007 Medicaid data in 13 states, found that 12.4% of children in foster care received antipsychotics, compared with 1.4% of Medicaid eligible children in general, according to Mr. Crystal, co-author of the study.
Bryan Samuels, head of the Administration on Children, Youth and Families, the agency within HHS that helps oversee the nation's foster children, is pushing states to adopt tougher rules on prescribing antipsychotics.
"The medications tend to be the stopgap measure," Mr. Samuels says. "We are making significant investments in medication that have limited evidence of effectiveness and rarely address the issues of trauma."
Write to Lucette Lagnado at lucette.lagnado@wsj.com
A version of this article appeared August 12, 2013, on page A1 in the U.S. edition of The Wall Street Journal, with the headline: U.S. Probes Psych Drug Use on Kids.












![[image]](./130812WSJUSProbesNeurolepticChildUse_files/NA-BX615_ANTISI_DV_20130811184045.jpg) Brandon Thibodeaux for The Wall Street Journal
Brandon Thibodeaux for The Wall Street Journal

![[image]](./130812WSJUSProbesNeurolepticChildUse_files/wsj_select_p_571x51.gif)
![[image]](./130812WSJUSProbesNeurolepticChildUse_files/wsj_select_p_377x107.gif)




![[image]](./130812WSJUSProbesNeurolepticChildUse_files/OB-YM911_NYSTOP_A_20130812095212(1).jpg)
![[image]](./130812WSJUSProbesNeurolepticChildUse_files/P1-BM663_NEWS_A_20130811184528.jpg)







.jpg)










Most Recommended
“It's more than disgraceful, it's...;”
“When you can't win with your...;”
“The core problem is that advocat...;”
“Disgraceful, what more can one say.;”
“He has completely conned the...;”